Moyamoyadisease (MMD) is a chronic cerebrovascular disease characterized by progressive stenosis of the large intracranial arteries and the secondary development of a collateral capillary meshwork from lenticulostriate artery hypertrophy, forming the characteristic "puff of smoke" appearance in angiographic imaging.1These vascular changes can manifest as hemorrhagic or ischemic disease.2,3The prevalence of MMD varies across geographic regions, with the largest numbers reported from East Asia.1,4The age of onset of this condition exhibits a bimodal distribution, with two peaks in early childhood and the 4th decade of life.5The pathophysiology of MMD is complex, multifactorial, and incompletely understood. However, upregulation of proangiogenic pathways, aberrant behavior of smooth muscle and endothelial progenitors, and autoimmune phenomena may play a role.6
虽然外科血管再生是站立的ard treatment for symptomatic MMD, it is associated with risks and limitations. Thus, noninvasive treatments are needed to improve outcomes. Cilostazol, a selective phosphodiesterase III inhibitor with antiplatelet, antithrombotic, and vasodilatory effects, has emerged as a potential therapy for MMD management. To provide a comprehensive overview of this topic, we conducted a systematic review of the early evidence on the safety, efficacy, and utilization of cilostazol in MMD patients. Additionally, to present a balanced and comprehensive view, we supplemented the systematic review findings with a comprehensive narrative synthesis of the literature on the surgical and medical management of MMD, considerations for the use of cilostazol, and its safety and efficacy in the general stroke population.
开云体育世界杯赔率
This systematic review was conducted in compliance with the PRISMA framework.7一个系统的文献检索是conducted to identify studies pertaining to the safety, efficacy, and utilization of cilostazol for the management of MMD. The search was performed using the PubMed database, employing the keywords "cilostazol" AND "moyamoya disease," along with their variations and relevant Medical Subject Headings terms, as outlined inTable 1. No limitations were applied during the search process, and the search was updated on June 5, 2023, to incorporate the most recent published evidence.
PubMed search strategy
| Search No. | Query | Details | Results* |
|---|---|---|---|
| 1 | Cilostazol OR "PDE3 Inhibitor" OR "PDE3 Inhibitors" OR "PDE 3 Inhibitor" OR "PDE 3 Inhibitors" OR "Phosphodiesterase 3 Inhibitor" OR "Phosphodiesterase 3 Inhibitors" | "cilostazol" [MeSH Terms] OR "cilostazol" [All Fields] OR "PDE3 Inhibitor" [All Fields] OR "PDE3 Inhibitors" [All Fields] OR "PDE 3 Inhibitor" [All Fields] OR "PDE 3 Inhibitors" [All Fields] OR "Phosphodiesterase 3 Inhibitor" [All Fields] OR "Phosphodiesterase 3 Inhibitors" [All Fields] | 6425 |
| 2 | Moyamoya OR "Moya Moya" | "moyamoya disease" [MeSH Terms] OR ("moyamoya" [All Fields] AND "disease" [All Fields]) OR "moyamoya disease" [All Fields] OR "moyamoya" [All Fields] OR "Moya Moya" [All Fields] | 5549 |
| 3 | Search nos. 1 AND 2 | ("cilostazol" [MeSH Terms] OR "cilostazol" [All Fields] OR "PDE3 Inhibitor" [All Fields] OR "PDE3 Inhibitors" [All Fields] OR "PDE 3 Inhibitor" [All Fields] OR "PDE 3 Inhibitors" [All Fields] OR "Phosphodiesterase 3 Inhibitor" [All Fields] OR "Phosphodiesterase 3 Inhibitors" [All Fields]) AND ("moyamoya disease" [MeSH Terms] OR ("moyamoya" [All Fields] AND "disease" [All Fields]) OR "moyamoya disease" [All Fields] OR "moyamoya" [All Fields] OR "Moya Moya" [All Fields]) | 8 |
MeSH = Medical Subject Headings.
Updated on June 5, 2023.
The eligibility criteria encompassed original studies conducted on human subjects, excluding review papers, case reports, editorials, and conference proceedings. Furthermore, the studies of interest were required to primarily focus on the assessment of cilostazol therapy in terms of its safety, efficacy, or utilization for the management of patients with MMD. Two authors (A.A. and R.C.R.) independently reviewed the search records and evaluated them for eligibility based on the aforementioned criteria.
One of the reviewers (A.A.) conducted the data extraction and narrative synthesis of the systematic review findings, incorporating all relevant data in the source publications. Another author (R.C.R.) reviewed the data independently for accuracy. To allow for a comprehensive scoping review, specific outcomes for data collection were not defined a priori. Due to the scoping nature of the review, formal risk of bias and certainty assessments were not applicable. Meta-analysis was not feasible owing to the considerable heterogeneity in design and reporting among the included studies.
Sections of this study pertaining to the surgical and medical management of MMD, as well as those presenting evidence about cilostazol in the general stroke population, were based on a narrative review of the literature that was identified through a nonsystematic search performed by the review team.
Surgical Treatment of MMD
Surgical revascularization using direct, indirect, or combined approaches is the main surgical treatment for symptomatic MMD.8Direct bypass is most commonly performed and has demonstrated immediate improvement in cerebral perfusion9,10and lower long-term risk of recurrent hemorrhagic and ischemic events, particularly in patients with hemorrhagic MMD presentations.3,11–17Direct bypass can nonetheless be technically challenging, with risks related to iatrogenic injury to the donor or recipient vessels, perioperative ischemia due to recipient vessel occlusion, intracerebral hemorrhage, and graft failure.17,18Complications such as cerebral hyperperfusion syndrome can also arise, especially with higher flow constructs.14,19
Indirect revascularization is technically less challenging, although it provides delayed revascularization and less robust collateral formation in adult-onset MMD patients compared with pediatric patients.11,20,21多党民主运动的患者接受间接搭桥可能better clinical outcomes than those who are untreated.11,22However, meta-analyses indicate higher recurrence of hemorrhagic and ischemic events after indirect versus direct bypass, although the quality of the available evidence on this topic is limited.11
Combined direct-indirect revascularization procedures incorporate the advantages of both approaches to achieve both immediate (direct) and long-term revascularization via the development of collaterals (indirect),23effectively preventing hemorrhagic and ischemic events in MMD patients.24–26A recent meta-analysis confirmed that direct or combined strategies offer better long-term stroke outcomes than indirect bypass in adult MMD patients.11However, the current literature is inconclusive regarding the benefits of a combined approach over direct revascularization.11,23,27,28
In the absence of definitive guidelines, the decision to perform surgery, its timing relative to symptoms/disease progression, and the revascularization strategy selected are often made on an individualized basis. The significant anesthetic risks in this population due to the intolerance of hypotension and hypoperfusion further highlight the surgical challenges and the advantage of consolidating such procedures in high-volume centers.
Medical Treatment of MMD
鉴于revascularizatio的风险和挑战n surgery, improved medical treatments are needed to lower the risk of future strokes in MMD patients. Because intraluminal thrombosis and embolism can cause ischemic symptoms, antiplatelet therapies are often used in this population to lower stroke risk.29,30Aspirin (cyclooxygenase inhibitor) and clopidogrel (P2Y12 inhibitor) are the most commonly prescribed antiplatelets for MMD, although other agents such as the phosphodiesterase inhibitors dipyridamole and increasingly cilostazol (particularly in Asia) are being utilized. Data on the effectiveness of antiplatelets for MMD are nonetheless mixed and often do not differentiate on the basis of the agents used. In a retrospective cohort of 1925 patients presenting with stroke from MMD, prehospital antiplatelet usage (including aspirin, clopidogrel, or cilostazol) was associated with better functional status on hospital admission, yielding higher odds of presenting with a modified Rankin Scale score of 0–1 (OR 1.52, 95% CI 1.11–2.09).31A comparison of two propensity score-matched subgroups yielded even larger effects for prehospital antiplatelet treatment as a predictor of better functional status on admission (OR 3.82, 95% CI 1.22–11.99). In another retrospective study of 243 hemodynamically stable MMD patients, Pang et el. assessed the outcomes associated with a variety of antiplatelet medications including aspirin, cilostazol, dipyridamole, or clopidogrel. Over a mean ± SD follow-up of 62 ± 43 months, they found no significant differences in hemorrhage or infarction between patients who received antiplatelets (121 [49.8%]) and controls, although longer antiplatelet use slightly increased the odds of improvement in ischemic symptoms (adjusted OR 1.02, 95% CI 1.01–1.03).32
这是不确定如果独自抗血小板治疗preventative benefits in MMD patients, and the optimal regimen and duration of treatment remain elusive. However, aspirin has a favorable safety profile in the perioperative setting and is commonly used as an adjunct to bypass surgery to improve outcomes. In a retrospective review of 74 combined revascularizations for MMD, preoperative aspirin use (52 procedures [70.3%]) was associated with a significantly lower rate of white thrombus formation at the anastomosis site and higher initial bypass patency compared with nonaspirin controls (22 procedures [29.8%]). This beneficial effect was seen whether it was stopped 3 days prior to surgery, the day of surgery, or continued postoperatively, likely due to the irreversible action of aspirin on platelets.33Unlike heparin anticoagulation, which can lower the risk of white thrombus formation, perioperative aspirin use did not increase the risk of hemorrhagic events.33
Cilostazol: a Pleiotropic Antiplatelet Medication
Cilostazol is a pleiotropic antiplatelet agent that may have a beneficial role in the medical treatment of MMD. It is a selective phosphodiesterase III inhibitor that has been used to manage symptomatic vascular claudication for over 2 decades.34This quinolinone derivative affects the cyclic nucleotide second messenger systems, including cGMP and more prominently cAMP, leading to antiplatelet, antithrombotic, and vasodilatory effects.34This wide spectrum of therapeutic characteristics makes cilostazol an appealing candidate for MMD management, as it can simultaneously target several critical pathophysiological pathways. Cilostazol can also have additive or synergistic effects when used with other medications, with a favorable safety profile. Although conventional dual antiplatelet therapy with aspirin and clopidogrel both lowers recurrent stroke risk and increases hemorrhage risk, adding cilostazol as a second agent to clopidogrel for high-risk patients with ischemic stroke maintains this stroke prevention effect without increasing the risks of hemorrhage.35This is thought to be achieved in part through synergistic enhancement of the effects of clopidogrel and pleiotropic effects such as those involved in vasodilation.35This combination therapy may also benefit individuals with lower cytochrome P450 2C19 function, a genetic polymorphism that reduces the efficacy of clopidogrel in patients with ischemic stroke.35,36
Cilostazol is administered orally in tablet form, with available dosages of 50 mg and 100 mg. For the FDA-approved indication for cilostazol therapy (intermittent claudication), the recommended dosage in adult patients is 100 mg every 12 hours.37类似的剂量已报告药品核准标示外uses of this medication. The safety and efficacy of cilostazol in the pediatric and adolescent populations have not been established. Monitoring of patients receiving cilostazol therapy includes periodic assessments of white blood cell and platelet counts. Additionally, it is important to evaluate patients for cardiac symptoms or the presence of a new systolic murmur after initiating cilostazol treatment, particularly in those individuals who are at a higher risk of experiencing serious cardiac complications.37
The synergistic and pleiotropic effects of cilostazol have also been demonstrated in relation to aspirin use, where it has been shown to reduce high on-aspirin platelet reactivity, a condition affecting up to 40% of individuals and leading to suboptimal platelet inhibition in response to aspirin.34,38,39This modulatory effect is mediated through inhibition of platelet multidrug resistance protein 4 and occurs independent of the cAMP pathway.34,38,39Compared to other routinely prescribed antiplatelet medications, the vasodilatory effects of cilostazol are also unique, with an increase in the protein kinase A signaling pathway (dependent on cAMP levels) that initiates a host of downstream changes including decreases in myosin light chain kinase activity and intracellular calcium levels in smooth muscles, as well as an increase in nitric oxide and modulation of the transmembrane potential.34,40,41
Cilostazol and Ischemic Stroke
西洛地唑的使用一般中风探索prevention offers insights into its potential role in MMD. Although aspirin is the current standard for long-term secondary stroke prevention, randomized controlled trials (RCTs) have demonstrated that cilostazol is comparable or superior to aspirin for this purpose. Over 2 decades ago, the Cilostazol Stroke Prevention Study (CSPS), which included 1052 patients randomized to cilostazol and placebo groups, demonstrated that long-term cilostazol use significantly reduced the recurrence of cerebral infarction compared to placebo and without significant adverse effects.42A follow-up study reported similar rates of secondary ischemic events between cilostazol and aspirin, with fewer patients experiencing hemorrhagic events with cilostazol treatment.43Mild adverse effects of medication, such as dizziness, diarrhea, and tachycardia, were nonetheless more prevalent with cilostazol.43Another prospective, multicenter, double-blind RCT from China randomly assigned 720 patients to receive aspirin (n = 360) or cilostazol (n = 360) after ischemic stroke.44This study yielded no significant differences in the annual rates of stroke recurrence between the two groups. However, the incidence of severe cerebral hemorrhage was significantly lower in the cilostazol group (0.28% vs 1.95%, relative risk 7.14, p = 0.038), suggesting a safer hemorrhagic risk profile for cilostazol after ischemic stroke.44A 2022 CSPS subgroup analysis also found that combined aspirin or clopidogrel and cilostazol dual therapy started at 15–180 days poststroke significantly reduced the longer term rates of ischemic stroke when compared with aspirin or clopidogrel monotherapy, without increasing hemorrhage risk.45These data, combined with the finding that the enhanced efficacy of traditional dual antiplatelet therapy (aspirin and clopidogrel) in preventing secondary stroke is limited to the first 21 days poststroke,46suggest that transition to cilostazol as part of a dual antiplatelet regimen, once outside the acute setting, may represent a preferred long-term stroke prevention strategy in high-risk patients. Overall, these data indicate that cilostazol is at least equivalent to aspirin in safety and efficacy for secondary stroke prevention and is likely to have an increasing role in standard combination therapies.
In addition to its long-term benefit, cilostazol has been studied in the acute poststroke setting where it is likely comparable to aspirin in both safety and efficacy.47In the Cilostazol in Acute Ischemic Stroke Treatment (CAIST) RCT with 458 patients, cilostazol demonstrated no significant differences in bleeding complications, cardiovascular events, adverse events, or drug discontinuation when administered during the acute poststroke phase (within 48 hours to 90 days) compared with aspirin.47Cilostazol may also prevent acute stroke progression, with data from an RCT with 510 patients showing no significant difference in stroke progression between cilostazol and standard acute stroke medical care including, but not limited to, aspirin, thienopyridines, or warfarin—depending on the patient’s specific indications.48
Aside from stroke prevention, emerging data suggest that cilostazol may improve neurological outcomes after ischemic stroke. Nakamura et al. reported a small-scale RCT of 76 patients in 2012, demonstrating that combined aspirin and cilostazol in the first 14 days after an ischemic stroke led to less neurological deterioration and more favorable functional status compared with aspirin alone.49Of note, the limited sample size of this study restricts the quality of the evidence derived from this pilot trial. Additionally, a multicenter prospective open-label study with 1201 patients comparing aspirin plus cilostazol to aspirin monotherapy administered within 48 hours of stroke symptoms showed no significant differences in short-term neurological deterioration between the two groups.50Further studies are thus needed to definitively assess the potential of cilostazol to impact neurological outcomes after stroke.
Systematic Review Findings: Efficacy and Utilization of Cilostazol in MMD Management
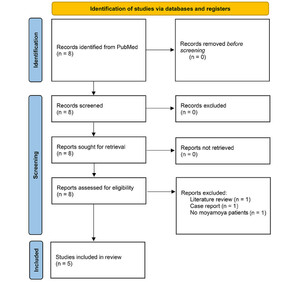
The study selection process (PRISMA flow diagram) is outlined inFig. 1. A total of 8 records were retrieved from the PubMed search, of which 5 studies remained eligible for inclusion in this review and are discussed below. Notably, only 1 study provided information regarding the adverse effect profile of cilostazol in MMD, while no studies were found that specifically focused on the pediatric population.

PRISMA flow diagram of the systematic literature search for studies pertaining to the safety, efficacy, and utilization of cilostazol for the management of moyamoya disease. Data added to the PRISMA template (from Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.BMJ. 2021;372:n717) under the terms of the Creative Commons Attribution (CC BY 4.0) License (https://creativecommons.org/licenses/by/4.0/).
Although RCTs are lacking, a growing body of studies has emerged that supports the use of cilostazol in the medical management of MMD. In 2016, Oki et al. performed a nationwide survey of antiplatelet treatment policies among stroke centers in Japan, including 330 neurology, neurosurgery, pediatrics, and rehabilitation departments involved in MMD management.51They reported a variable rate of antiplatelet administration (51.3% ± 29.8%) for MMD among participating facilities, but cilostazol was the second most commonly prescribed antiplatelet treatment (62.5%) after aspirin (76.3%).51Cilostazol treatment rates were notably higher among experienced departments than less experienced counterparts (70.3% vs 56.9%), although no formal statistical significance was reported.51In a large population-based study from Korea, cilostazol was prescribed to more than 52% of the MMD patients who underwent antiplatelet treatment in 2018: it was the most common antiplatelet agent for this population, with a more than 9-fold increase in its prescription rate since 2002.52In this retrospective study of 25,978 newly diagnosed MMD patients, multivariate analysis yielded significant increases in survival with antiplatelet therapy, while cilostazol demonstrated the largest decrease in mortality (hazard ratio 0.78, 95% CI 0.68–0.90) compared with aspirin, clopidogrel, combination therapy, and other antiplatelets.52Further analysis of subgroups based on age (< 45 vs ≥ 45 years old) and history of ischemic or hemorrhagic stroke showed that cilostazol was the only antiplatelet regimen that significantly reduced the risk of mortality in all four subcohorts.52
In another nonrandomized prospective study, Chiba et al. utilized PET to assess the cerebral blood flow (CBF) changes of symptomatic MMD patients undergoing clopidogrel or cilostazol stand-alone medical therapy.53They followed 68 patients without misery perfusion over a 2-year period. Patients undergoing cilostazol treatment yielded significant improvements in CBF compared with baseline, versus insignificant changes in the clopidogrel group.53Specifically, a total of 10 patients yielded CBF improvement, defined as CBF > 1.088 of baseline in at least one middle cerebral artery region, of whom 9 were from the cilostazol subgroup.53However, treatment assignment in this study was based on age, with patients younger than 50 years receiving cilostazol and others receiving clopidogrel, allowing for treatment to be switched in cases of intolerance. Therefore, the two subgroups differed significantly in terms of age, with higher rates of diabetes and lower rates of transient ischemic attack at baseline in the clopidogrel group. In another study performed by the same group, Ando et al. compared the effects of cilostazol and clopidogrel on neuropsychological and cerebral perfusion parameters.54The cilostazol group demonstrated significantly more improvements in cognitive function at 2-year follow-up. Specifically, 9/36 (25%) patients in the cilostazol group showed improved CBF and 14/36 (38.9%) had significant cognitive improvements, constituting 93.3% of all patients with cognitive benefits. In contrast, most patients in the clopidogrel group (29/30 [96.7%]) showed neither cognitive nor CBF improvements.54Even after controlling for CBF improvements, cilostazol treatment remained significantly associated with improved cognitive function, suggesting pleiotropic effects aside from restored perfusion.54This effect is supported by a previous population-based study that demonstrated a dose-dependent decrease in all-cause dementia incidence with cilostazol treatment in the general population, although cognitive benefits in the stroke population are debated.55,56
Although cilostazol has only been studied as a conservative management option or prior to revascularization surgery, in theory the same principles underlying its benefits during this period may also apply postoperatively. Specifically, cilostazol appears to be a good candidate for the maintenance antiplatelet regimen after the acute postoperative period, and empirical evidence is needed to determine its safety and potential efficacy in this setting.
Safety of Cilostazol in the General Stroke Population: Implications for MMD
For any antiplatelet therapy to be considered for the management of MMD, its benefits must offset any increased risk of intracranial hemorrhage. The evidence on the safety of cilostazol treatment in the MMD population is currently limited. It is important to note that the safety data derived from the general stroke population should not be directly extrapolated to the MMD population, as each population may exhibit distinct characteristics. However, considering the paucity of safety data specific to MMD, the evidence derived from the general stroke population can provide preliminary insights until further evidence becomes available. These findings may guide future studies within the MMD population, although caution should be exercised when generalizing these findings across populations.
In the US, cilostazol is the only FDA-approved drug for the treatment of vascular claudication in peripheral arterial disease based on class 1A evidence.57However, clinical trials have shown promising benefits for cilostazol in the secondary prevention of ischemic and hemorrhagic stroke, with lower intracerebral and other major hemorrhage rates than other antiplatelet agents such as aspirin or clopidogrel.57–63Nevertheless, these trials had limitations such as open-label design, premature termination, loss to follow-up, lack of functional or cognitive outcome data, and near exclusive patient enrollment in Asia with a male predominance.57This poor generalizability has led to no significant changes in clinical practice guidelines regarding cilostazol administration in stroke patients outside of Asia.57
Cilostazol has nonetheless shown a reasonably benign safety profile in clinical trials when used in patients with noncardioembolic strokes.57The most common adverse effects in this setting include heart palpitations, dizziness, headaches, rhinorrhea, and diarrhea, which are primarily mild to moderate and transient and can be attenuated by starting the medication at a lower dose and titrating up as tolerated.53,57,64Regardless, some patients randomly assigned to cilostazol in these trials have discontinued the drug due to these adverse effects.57Among the few clinical studies carried out in an MMD population, Chiba et al. reported that 17/54 patients (31%) experienced cilostazol adverse effects including diarrhea, headaches, runny nose, and dizziness, requiring a change to clopidogrel per study protocol within 4 weeks of treatment.53Other cardiovascular adverse effects include tachycardia, tachyarrhythmia, and hypotension.65Cilostazol has an FDA boxed warning with contraindication in patients with class III–IV heart failure because of potential exacerbations of angina or myocardial infarction, likely secondary to vasodilatory effects.57Although the Cilostazol Safety Database has not revealed any increase in cardiac-related mortality, it should be used cautiously in Western populations with a higher coronary artery disease prevalence than Asian counterparts.66The plasma concentration of cilostazol increases with severe hepatic and renal impairment, when taken in conjunction with other drugs metabolized by CYP3A4 or CYP2C19, or when taken with high-fat meals; thus, caution is needed in these settings.57Finally, although it is well tolerated when used with aspirin or clopidogrel, cilostazol is associated with increased bleeding risk when used in conjunction with the anticoagulant warfarin.50,57
Conclusions
Despite the proven benefits of revascularization surgery as the single available treatment for MMD, better medical therapies are needed to lower the risk of brain ischemia. Cilostazol targets critical pathways in the pathophysiology of MMD and the evidence corroborates its benefits in terms of decreased mortality, improved cerebral perfusion, and cognitive function. However, due to the small number of studies and lack of RCTs, these findings should be interpreted with caution. Subgroups of patients need to be identified who can safely undergo medical management in lieu of revascularization surgery or to improve surgical outcomes. Cilostazol seems to have a benign safety profile in the general stroke population, but future studies are needed to definitively assess its safety and efficacy for management of MMD, especially in Western populations.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Russin, Abedi, Sizdahkhani. Acquisition of data: Abedi, Rennert. Analysis and interpretation of data: Russin, Abedi, Nguyen. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Russin. Administrative/technical/material support: Abedi, Nguyen. Study supervision: Russin, Abedi.
Supplemental Information
Previous Presentations
A digital poster was presented at the American Association of Neurological Surgeons Annual Meeting, Los Angeles, CA, April 21–24, 2023.
Data Availability
Protocol and data extraction form are available upon reasonable request.
References
-
1 ↑
KimJS.Moyamoya disease: epidemiology, clinical features, and diagnosis.J Stroke.2016;18(1):2–11.
-
2 ↑
XieA,LuoL,DingY,LiG.Ischemic and hemorrhagic moyamoya disease in adults: CT findings.Int J Clin Exp Med.2015;8(11):21351–21357.
-
4 ↑
HuangS,GuoZN,ShiM,YangY,饶M.Etiology and pathogenesis of moyamoya disease: an update on disease prevalence.Int J Stroke.2017;12(3):246–253.
-
5 ↑
KimJS.Moyamoya disease and syndrome. In:CaplanLR,BillerJ,LearyMC,et al.eds.Primer on Cerebrovascular Diseases.2nd ed.Academic Press;2017:561-566.
-
6 ↑
BangOY,FujimuraM,KimSK.The pathophysiology of moyamoya disease: an update.J Stroke.2016;18(1):12–20.
-
7 ↑
PageMJ,McKenzieJE,BossuytPM,et al.The PRISMA 2020 statement: an updated guideline for reporting systematic reviews.BMJ.2021;372(71):n71.
-
8 ↑
Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis; Health Labour Sciences Research Grant for Research on Measures for Infractable Diseases.Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis).神经医学Chir(东京).2012;52(5):245–266.
-
9 ↑
LiZ,ZhouP,XiongZ,et al.Perfusion-weighted magnetic resonance imaging used in assessing hemodynamics following superficial temporal artery-middle cerebral artery bypass in patients with moyamoya disease.Cerebrovasc Dis.2013;35(5):455–460.
-
10 ↑
ChenY,XuW,GuoX,et al.CT perfusion assessment of moyamoya syndrome before and after direct revascularization (superficial temporal artery to middle cerebral artery bypass).Eur Radiol.2016;26(1):254–261.
-
11 ↑
NguyenVN,MotiwalaM,ElarjaniT,et al.Direct, indirect, and combined extracranial-to-intracranial bypass for adult moyamoya disease: an updated systematic review and meta-analysis.Stroke.2022;53(12):3572–3582.
-
12
NguyenVN,ParikhKA,MotiwalaM,et al.Surgical techniques and indications for treatment of adult moyamoya disease.Front Surg.2022;9:966430.
-
13
MiyamotoS,AkiyamaY,NagataI,et al.Long-term outcome after STA-MCA anastomosis for moyamoya disease.Neurosurg Focus.1998;5(5):e5.
-
14 ↑
MesiwalaAH,SviriG,FatemiN,BritzGW,NewellDW.Long-term outcome of superficial temporal artery-middle cerebral artery bypass for patients with moyamoya disease in the US.Neurosurg Focus.2008;24(2):E15.
-
15
MiyamotoS,YoshimotoT,HashimotoN,et al.Effects of extracranial-intracranial bypass for patients with hemorrhagic moyamoya disease: results of the Japan Adult Moyamoya Trial.Stroke.2014;45(5):1415–1421.
-
16
LiQ,GaoY,XinW,et al.Meta-analysis of prognosis of different treatments for symptomatic moyamoya disease.World Neurosurg.2019;127:354–361.
-
17 ↑
JeonJP,KimJE,ChoWS,BangJS,SonYJ,OhCW.Meta-analysis of the surgical outcomes of symptomatic moyamoya disease in adults.J Neurosurg.2018;128(3):793–799.
-
18 ↑
BaajAA,AgazziS,SayedZA,ToledoM,SpetzlerRF,van LoverenH.Surgical management of moyamoya disease: a review.Neurosurg Focus.2009;26(4):E7.
-
19 ↑
FujimuraM,KanetaT,MugikuraS,ShimizuH,TominagaT.Temporary neurologic deterioration due to cerebral hyperperfusion after superficial temporal artery-middle cerebral artery anastomosis in patients with adult-onset moyamoya disease.Surg Neurol.2007;67(3):273–282.
-
20 ↑
HoukinK,NakayamaN,KurodaS,IshikawaT,NonakaT.How does angiogenesis develop in pediatric moyamoya disease after surgery? A prospective study with MR angiography.Childs Nerv Syst.2004;20(10):734–741.
-
21 ↑
MizoiK,KayamaT,YoshimotoT,NagamineY.Indirect revascularization for moyamoya disease: is there a beneficial effect for adult patients?Surg Neurol.1996;45(6):541–549.
-
22 ↑
HanDH,NamDH,OhCW.Moyamoya disease in adults: characteristics of clinical presentation and outcome after encephalo-duro-arterio-synangiosis.Clin Neurol Neurosurg.1997;99(suppl 2):S151–S155.
-
23 ↑
FiaschiP,ScalaM,PiatelliG,et al.Limits and pitfalls of indirect revascularization in moyamoya disease and syndrome.Neurosurg Rev.2021;44(4):1877–1887.
-
24 ↑
ChoWS,KimJE,KimCH,et al.Long-term outcomes after combined revascularization surgery in adult moyamoya disease.Stroke.2014;45(10):3025–3031.
-
25
JiangH,YangH,NiW,et al.Long-term outcomes after combined revascularization surgery in adult hemorrhagic moyamoya disease.World Neurosurg.2018;116:e1032–e1041.
-
26 ↑
RashadS,FujimuraM,NiizumaK,EndoH,TominagaT.Long-term follow-up of pediatric moyamoya disease treated by combined direct-indirect revascularization surgery: single institute experience with surgical and perioperative management.Neurosurg Rev.2016;39(4):615–623.
-
27 ↑
SunH,WilsonC,OzpinarA,et al.Perioperative complications and long-term outcomes after bypasses in adults with moyamoya disease: a systematic review and meta-analysis.World Neurosurg.2016;92:179–188.
-
28 ↑
SunJ,LiZY,ChenC,LingC,LiH,WangH.Postoperative neovascularization, cerebral hemodynamics, and clinical prognosis between combined and indirect bypass revascularization procedures in hemorrhagic moyamoya disease.Clin Neurol Neurosurg.2021;208:106869.
-
29 ↑
JeonC,YeonJY,JoKI,HongSC,KimJS.Clinical role of microembolic signals in adult moyamoya disease with ischemic stroke.Stroke.2019;50(5):1130–1135.
-
31 ↑
OnozukaD,HagiharaA,NishimuraK,et al.Prehospital antiplatelet use and functional status on admission of patients with non-haemorrhagic moyamoya disease: a nationwide retrospective cohort study (J-ASPECT study).BMJ Open.2016;6(3):e009942.
-
32 ↑
PangCH,ChoWS,KangHS,KimJE.Benefits and risks of antiplatelet medication in hemodynamically stable adult moyamoya disease.Sci Rep.2021;11(1):19367.
-
33 ↑
KanamoriF,ArakiY,YokoyamaK,et al.Effects of aspirin and heparin treatment on perioperative outcomes in patients with moyamoya disease.Acta Neurochir (Wien).2021;163(5):1485–1491.
-
34 ↑
KherallahRY,KhawajaM,OlsonM,AngiolilloD,BirnbaumY.Cilostazol: a review of basic mechanisms and clinical uses.Cardiovasc Drugs Ther.2022;36(4):777–792.
-
35 ↑
HoshinoH,ToyodaK,OmaeK,et al.Dual antiplatelet therapy using cilostazol with aspirin or clopidogrel: subanalysis of the CSPS.com trial.Stroke.2021;52(11):3430–3439.
-
36 ↑
PanY,ChenW,XuY,et al.Genetic polymorphisms and clopidogrel efficacy for acute ischemic stroke or transient ischemic attack: a systematic review and meta-analysis.Circulation.2017;135(1):21–33.
-
38 ↑
AlemannoL,MassimiI,KlausV,et al.Impact of multidrug resistance protein-4 inhibitors on modulating platelet function and high on-aspirin treatment platelet reactivity.Thromb Haemost.2018;118(3):490–501.
-
39 ↑
ZhangJW,LiuWW,McCaffreyTA,et al.Predictors of high on-aspirin platelet reactivity in elderly patients with coronary artery disease.Clin Interv Aging.2017;12:1271–1279.
-
40 ↑
ManickavasagamS,YeY,LinY,et al.The cardioprotective effect of a statin and cilostazol combination: relationship to Akt and endothelial nitric oxide synthase activation.Cardiovasc Drugs Ther.2007;21(5):321–330.
-
41 ↑
NishiokaK,NishidaM,AriyoshiM,et al.Cilostazol suppresses angiotensin II-induced vasoconstriction via protein kinase A-mediated phosphorylation of the transient receptor potential canonical 6 channel.Arterioscler Thromb Vasc Biol.2011;31(10):2278–2286.
-
42 ↑
GotohF,TohgiH,HiraiS,et al.Cilostazol stroke prevention study: a placebo-controlled double-blind trial for secondary prevention of cerebral infarction.J Stroke Cerebrovasc Dis.2000;9(4):147–157.
-
43 ↑
ShinoharaY,KatayamaY,UchiyamaS,et al.Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomised non-inferiority trial.Lancet Neurol.2010;9(10):959–968.
-
44 ↑
HuangY,ChengY,WuJ,et al.Cilostazol as an alternative to aspirin after ischaemic stroke: a randomised, double-blind, pilot study.Lancet Neurol.2008;7(6):494–499.
-
45 ↑
ToyodaK,OmaeK,HoshinoH,et al.Association of timing for starting dual antiplatelet treatment with cilostazol and recurrent stroke: a CSPS.com trial post hoc analysis.Neurology.2022;98(10):e983–e992.
-
46 ↑
PanY,ElmJJ,LiH,et al.Outcomes associated with clopidogrel-aspirin use in minor stroke or transient ischemic attack: a pooled analysis of Clopidogrel in High-Risk Patients With Acute Non-Disabling Cerebrovascular Events (CHANCE) and Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke (POINT) trials.JAMA Neurol.2019;76(12):1466–1473.
-
47 ↑
LeeYS,BaeHJ,KangDW,et al.Cilostazol in Acute Ischemic Stroke Treatment (CAIST Trial): a randomized double-blind non-inferiority trial.Cerebrovasc Dis.2011;32(1):65–71.
-
48 ↑
ShimizuH,TominagaT,OgawaA,et al.Cilostazol for the prevention of acute progressing stroke: a multicenter, randomized controlled trial.J Stroke Cerebrovasc Dis.2013;22(4):449–456.
-
49 ↑
NakamuraT,TsurutaS,UchiyamaS.Cilostazol combined with aspirin prevents early neurological deterioration in patients with acute ischemic stroke: a pilot study.J Neurol Sci.2012;313(1-2):22–26.
-
50 ↑
AokiJ,IguchiY,UrabeT,et al.Acute aspirin plus cilostazol dual therapy for noncardioembolic stroke patients within 48 hours of symptom onset.J Am Heart Assoc.2019;8(15):e012652.
-
51 ↑
OkiK,KatsumataM,IzawaY,TakahashiS,SuzukiN,HoukinK.Trends of antiplatelet therapy for the management of moyamoya disease in Japan: results of a nationwide survey.J Stroke Cerebrovasc Dis.2018;27(12):3605–3612.
-
52 ↑
SeoWK,KimJY,ChoiEH,et al.Association of antiplatelet therapy, including cilostazol, with improved survival in patients with moyamoya disease in a nationwide study.J Am Heart Assoc.2021;10(5):e017701.
-
53 ↑
ChibaT,SettaK,ShimadaY,et al.Comparison of effects between clopidogrel and cilostazol on cerebral perfusion in nonsurgical adult patients with symptomatically ischemic moyamoya disease: subanalysis of a prospective cohort.J Stroke Cerebrovasc Dis.2018;27(11):3373–3379.
-
54 ↑
AndoS,TsutsuiS,MiyoshiK,et al.Cilostazol may improve cognition better than clopidogrel in non-surgical adult patients with ischemic moyamoya disease: subanalysis of a prospective cohort.Neurol Res.2019;41(5):480–487.
-
55 ↑
TaiSY,ChienCY,ChangYH,YangYH.Cilostazol use is associated with reduced risk of dementia: a nationwide cohort study.Neurotherapeutics.2017;14(3):784–791.
-
56 ↑
LimJS,KwonSU,YuKH,et al.Cilostazol and probucol for cognitive decline after stroke: a cognitive outcome substudy of the PICASSO trial.J Stroke.2021;23(1):128–131.
-
57 ↑
de HavenonA,ShethKN,MadsenTE,et al.Cilostazol for secondary stroke prevention: history, evidence, limitations, and possibilities.Stroke.2021;52(10):e635–e645.
-
58
NomaK,HigashiY.Cilostazol for treatment of cerebral infarction.专家知道Pharmacother.2018;19(15):1719–1726.
-
59
UchiyamaS,ToyodaK,OmaeK,et al.Dual antiplatelet therapy using cilostazol in patients with stroke and intracranial arterial stenosis.J Am Heart Assoc.2021;10(20):e022575.
-
60
LinMP,MeschiaJF,GopalN,et al.Cilostazol versus aspirin for secondary stroke prevention: systematic review and meta-analysis.J Stroke Cerebrovasc Dis.2021;30(3):105581.
-
61
TanCH,WuAG,SiaCH,et al.Cilostazol for secondary stroke prevention: systematic review and meta-analysis.Stroke Vasc Neurol.2021;6(3):410–423.
-
62
ToyodaK,UchiyamaS,YamaguchiT,et al.Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial.Lancet Neurol.2019;18(6):539–548.
-
63 ↑
LiberopoulosEN,NonniAB,TsianosEV,ElisafMS.Possible ranitidine-induced cholestatic jaundice.Ann Pharmacother.2002;36(1):172.
-
64 ↑
McHutchisonC,BlairGW,AppletonJP,et al.Cilostazol for secondary prevention of stroke and cognitive decline: systematic review and meta-analysis.Stroke.2020;51(8):2374–2385.
-
65 ↑
KimBJ,KwonSU,ParkJH,et al.Cilostazol versus aspirin in ischemic stroke patients with high-risk cerebral hemorrhage: subgroup analysis of the PICASSO trial.Stroke.2020;51(3):931–937.
-
66 ↑
KimSM,JungJM,KimBJ,LeeJS,KwonSU.Cilostazol mono and combination treatments in ischemic stroke: an updated systematic review and meta-analysis.Stroke.2019;50(12):3503–3511.