Firstcoined in 1787, the term “meningeal lymphatics” has been used to describe structures that drain the CSF and interstitial space to the peripheral lymphatic system.1–4These structures constitute the perisinusal vessels that have been recently redescribed in mouse and other vertebrates.5,6However, evidence for corresponding structures in humans is not conclusive. Anatomically and histologically, the most similar structures to the perisinusal lymphatic vessels in the human brain are intradural vascular channels (IVCs) in the parasagittal dura mater, which were previously deemed distinct from meningeal lymphatics.7
Arachnoid granulations (AGs) are critical components of the CSF reabsorption pathway.8,9Here we examine the morphological and immunohistochemical features of AGs and IVCs in postmortem formalin-fixed and fresh human cadaveric specimens, and we identify a subset of AGs that connect to the venous circulation via IVCs. These IVCs stain positively with markers of lymphatic vasculature.
开云体育世界杯赔率
Consent was obtained from the institutional review board at the University of Virginia for the harvest of fresh tissue from cadavers during rapid autopsy.
Morphological Study
Eight formalin-fixed, adult cadaveric heads with no intracranial or extracranial pathology were prepared, dissected, and examined bilaterally as previously described.10We studied the number and size (length and width) of the AGs, their distance from glabella and the edge of thesuperior sagittal sinus(SSS), and their relationships with the IVCs and dural layers. The measurements were taken with a digital meter (Fig. 1).The dissections were performed under the operating microscope (OPMI/Neuro NC4; Carl Zeiss) (Figs. 2–4) and dissecting scope (Zeiss Stemi 305) (Figs. 5and6).The samples were photographed by a digital camera (Canon EOS 5D Mark IV).

The distribution and location of the AGs and the opening points into the SSS. Zero millimeters is the most anterior point of the SSS. Thestarsindicate the most anteromedial edge of each AG. Figure is available in color online only.

Dissection of the formalin-fixed cadavers.A:The middle one-third of the SSS on the left side was exposed.B:Enlarged view of thegreen squarein panel A, which shows the relationship of the lateral lacunae, middle meningeal artery (Meningeal Media A.), and intradural channels.C:The dura was reflected to expose the dural-arachnoid cohesion that is the CSF pathway from subarachnoid space to the AGs.D:The outer layer of the dura has been peeled away to expose the intradural AGs.E:In a higher magnification of panel D, the intradural channels surrounding the AGs were exposed.F:The outer layer of the parasagittal dura and the roof of the SSS were removed to observe the relationship of the AGs with lateral lacunae and the SSS.G:Enlarged view of panel F. The interdural and intradural AGs were exposed. A. = artery; Sag. = sagittal; Sup. = superior. Original magnification ×4 (B, E, G). Figure is available in color online only. Copyright Kaan Yağmurlu. Published with permission.

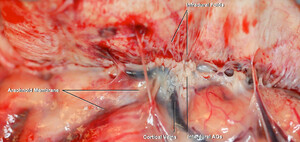
A:Removal of the blue silicone within the SSS reveals the openings of the lateral venous lacunae, cortical veins, and AGs.B:Enlarged view of the area within thegreen squarein panel A, in which the intradural AGs and lateral lacunae open into the superolateral wall of the sinus.C:Enlargement of the openings into the floor of the SSS where theyellow squareis located in panel A.D:Enlarged view showing the dura and intradural AGs, which were reflected to expose the adhesion and opening of the interdural AGs into the inferolateral wall and floor of the sinus. Original magnification ×4 (B), ×4 (C), ×8 (D). Figure is available in color online only. Copyright Kaan Yağmurlu. Published with permission.

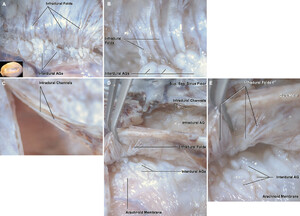
Fresh human autopsy material was imaged using a digital camera. The arachnoid membrane adheres to the interdural AGs and passes through the inner layer of the dura by passing through the infradural folds. Some branches of the cortical veins supply the dura. Figure is available in color online only.

A:The same specimen as depicted inFig. 4, but this was photographed under the dissecting microscope. The interdural AGs pass through the infradural folds to reach the intradural AGs.B:Enlarged view of panel A.C:The intradural channels within the inner and outer dural layers have been exposed.D:The relationships between the arachnoid membrane, interdural AGs, infradural folds, intradural AGs, and channels were exposed.E:Enlarged view of panel D. The infradural folds extend laterally from the midline. Sag. = sagittal; Sup. = superior. Original magnification ×8 (B, E). Figure is available in color online only.

A:The unroofing of the SSS exposes the sinus floor and intradural channels that run along the sinus beside the AGs.B:The intradural channels around the AG and along the sinus floor were seen. Sag. = sagittal. Figure is available in color online only.
Microscopic and Immunostaining Study
的日冕部分中间的三分之二SSS and adjacent structures, including the dura and brain, were obtained from 2 fresh adult human rapid autopsy samples with no cranial pathology. Tissue was fixed in 4% paraformaldehyde for 48 hours and then paraffin processed using standard techniques. Sections were cut at 10 µm, dewaxed, and rehydrated through sequential incubation in xylenes and graded alcohol baths. Antigen retrieval was performed by heating buffer (10 mM Tris, 1 mM EDTA, pH 9) to 80°C, adding slides, and allowing them to cool to room temperature. The sections were blocked in 2% normal goat serum and 0.1% Tween in phosphate-buffered saline for 1 hour at room temperature and then incubated overnight at 4°C in primary antibodies diluted in blocking buffer. The tissue was labeled with lineage-specific markers to identify specific cell types. To identify lymphatic tissue, the Lyve-1 and podoplanin (PDPN) antibodies were used. To label hematopoietic lineages, antibody against CD45 was used. The tissue was labeled with anti–Lyve-1 antibodies (1:400; Sigma-Aldrich USA); anti-PDPN antibody (1:100, mouse monoclonal, D2–40; BioLegend); and an anti-CD45 antibody (1:150, rabbit polyclonal, ab10558; Abcam) in phosphate-buffered saline with Tween overnight at 4°C.11Nuclei were counterstained with DAPI (1:100, Prolong Gold Antifade; ThermoFisher).
Results
Morphological Analysis of SSS and Openings
AGs can be divided into intradural and interdural groups based on their positions and relation to the dural layers. A subset of the interdural AGs adhere to the arachnoid membrane and cross the infradural folds of the dura’s inner layer to meet with the intradural AGs and IVCs. Some of the interdural AGs open directly into the inferolateral wall or floor of the SSS. Intradural AGs are located within the dural leaflets and may directly open into the SSS (Figs. 1–3.).CSF is thought to return to the venous circulation using AGs.
The most anterior opening into the SSS was 88.44 mm from the glabella. Structures that make connection with the SSS include cortical veins, lacunae, and AGs. The most common location of the opening into the SSS was in the middle one-third of the SSS, at a distance between 100 and 250 mm from the glabella. Most venous vessels and intradural AGs open into the superolateral wall of the SSS, whereas the interdural AGs open into the inferolateral wall and the floor of the SSS (Figs. 2and3.).The total number of the openings into the SSS, including the AGs and lateral lacunae and cortical veins, was 45 ± 5.62 per head. The mean number of the AGs in the examined specimens was 6 ± 1.30 per head. The mean anteroposterior lengths of the AGs were 17.16 ± 8.46 mm (range 4.53–30.96 mm) and 16.55 ± 9.18 mm (range 3.86–35.69 mm) on the right and left sides, respectively. The widths (mediolateral) of the AGs were 8.20 ± 3.19 mm (range 2.89–15.04 mm) and 8.26 ± 2.41 mm (range 3.15–13.08 mm) on the right and left sides, respectively.
Immunostaining AGs and Intradural Channels
Both interdural and intradural AGs were immunostained with lymphatic vessel (PDPN, Lyve-1) and immune cell (CD45) biomarkers (Figs. 7–9).的一个子集interdural AGs不make direct contact with the SSS, a network of IVCs seems to provide a bridge to the sinus via venous lacunae. These tubular structures resemble vessels and demonstrate immunostaining with markers of immune cells (CD45) and lymphatic vascular cells (Lyve-1, PDPN).7These tubular conduits, given the immunostaining patterns and the anatomical connections between AGs and the SSS, may serve as a conduit for CSF or interstitial fluid reabsorption into the venous circulation (Figs. 7–9).

疣状旁矢状面的冠状的硬脑膜的凹陷ment in a fresh human specimen coronal section. H&E-stained sections reveal that the intradural AGs invaginate into the dura, and the intradural channels are lined with endothelium (tissue outlined by theyellow squarein panel A is enlarged in panel B). Original magnification ×2.5 (A), ×10 (B).Figure is available in color online only.

A:Coronal section of the SSS and parasagittal dura were immunostained with Lyve-1 (green), PDPN (red), and DAPI (blue).B:Higher magnification of the parasagittal dura indicates PDPN (green) and Lyve-1 (red)染色,但缺乏渠道。原始magnification ×64 (B). Figure is available in color online only.

The interdural and intradural AGs and infradural folds were exposed. The capsule of the AG stained with the panvascular marker CD45 (red) and with DAPI (blue).The intradural and interdural AGs stained with both PDPN (green) and CD45. The interdural AGs pass through the infradural folds to connect with intradural AGs. Figure is available in color online only.
Discussion
In mammals the nasal mucosa, cribriform plate, and the arachnoid villi are pathways for CSF outflow.12–14Other possible drainage routes are the Virchow-Robin space around the arteries, the veins, and the cranial and spinal nerves.12,14,15In newborn animals, because the arachnoid villi are not well developed, theolfactory bulb, cribriform plate, and nasal mucosa are considered significant routes of CSF drainage.12,14Although most of the studies regarding CSF and interstitial fluid drainage from the CNS are from animal experiments, there are significant differences between animals and humans in CSF drainage pathways. In most animals, up to 50% of CSF drains to cervical lymph nodes. In contrast, the majority of CSF in adult humans seems to drain directly into venous blood via arachnoid villi and granulation.14,16–18The arachnoid villi are postulated to be the major CSF outflow pathway in humans. AGs are the herniation of the arachnoid membrane that protrudes through the dura mater and into the lateral lacunae and venous sinuses to drain the CSF to the brain’s venous system.12The AGs represent a fibrotic degenerative form of the arachnoid villi due to their fibrotic structures, insufficient cellularity, and less efficiency.13
Others have divided AGs into vascular and nonvascular subtypes.13The nonvascular AGs do not have a vein directly open into venous sinuses, and play a significant role in regulating intracranial pressure. Vascular AGs are centered on a vein and are associated with the point of entry of a cortical vein into the dural sinus or lateral lacunae, and not the vicinity of sinuses.8,9,19We further divided AGs into median and paramedian groups. We observed that most lateral AGs are located 5.04 mm from the edge of the SSS. Some intradural AGs are located next to the sinus but lack protrusions or openings directly into the sinus.
The venous system is anatomically separated into the lateral lacunae and the SSS. It is noted that the lateral lacunae are the meshwork of veins and granular plexus formed by the anastomosis of the terminal arborizations of the meningeal veins rather than being a protrusion of the SSS.7Additionally, one study reported that the lateral lacunae render a direct pathway to the venous system, which would allow for the outflow of CSF from AGs to the venous system. It was noted that the AGs are mostly located on the floor of the lateral lacunae and that there is direct connectivity of AGs with SSS.20There have been different findings in the literature about the distribution of the AGs along with the SSS or venous lacunae. One study found that AGs opening into SSS is a rare phenomenon, which corresponds to our findings. Another study (in 35 human cadaveric specimens) found that the opening of the AGs into the SSS occurs quite frequently.
In 8 formalin-fixed cadaveric heads, we found that the total number of the openings into the SSS, including the AGs, lateral lacunae, and cortical veins, was 45 ± 5.62 per head. The mean number of intradural AGs was 6 ± 1.30 per head. Some of the AGs drain into the lateral lacunae via the IVCs instead of draining directly into the SSS. One study has claimed that some of the IVCs open directly into the SSS without any valves.7We did not find any direct openings from the IVCs into the SSS, although some IVCs run around and along with the SSS. On the other hand, we found that some IVCs open directly into the lateral lacunae, which have a lower intravascular pressure than the SSS. Although the histological connection between the lateral venous lacunae and intradural channels is well known, the CSF traffic between the AGs and intradural channels remains uncertain due to ethical issues around human testing—these structures do not appear to have equivalents in rodent models frequently used for study. Notably, IVCs have previously been demonstrated to stain positive for factor VIII, as have the lateral lacunae and SSS. This finding suggests that the intradural channels are extensions of the venous circulation. Complicating this conclusion is the observation that lymphatic endothelial cells also stain positive for factor VIII. Therefore, a lymphatic origin of the IVCs, lacunae, and AGs cannot be excluded. Lyve-1 and PDPN are the most sensitive and specific markers currently available for the lymphatic endothelial cell. The IVCs isolated in our study stain positive for both markers, as well as CD45.
Evidence for the presence of lymphatic structures in the CNS of humans is sparse. One recent study demonstrated the presence of dural structures with molecular signatures of lymphatics, capable of draining CSF to the deep cervical lymph nodes in humans.5Another study used MRI and various contrast agents to demonstrate the presence of perisinusal lymphatic vessels in human and nonhuman primates at the same location as in rodents.21Our study demonstrates the presence of tubular IVCs, which stain positively with markers of lymphatic vessels, connecting the lateral interdural AGs with the venous circulation. We did not identify perisinusal structures in our specimens as has been described in rodents.
The known primary function of the AGs is CSF absorption from subarachnoid space and drainage to the venous system. AGs also play an essential role in the intake of the CSF’s waste products of neuronal metabolism. This function raises the possibility that AGs may function as a component of the CNS lymphatic drainage, serving as a surrogate for lymph nodes in the CNS. We speculate that the relationship of the AGs to the adherent arachnoid membranes suggests that some AGs may function in CSF egress, whereas others may subserve lymphatic drainage. This hypothesis remains to be studied and rigorously tested. These findings have broad clinical implications, especially for the mechanism of entry and exit of immune cells to the CNS, mechanisms of cancer metastasis, and alterations in clearance of macromolecules that may lead to neurodegenerative diseases.11,22
This study has several limitations. The sample of dura studied did not include tissue from different regions of the dura mater. Instead, due to limitations associated with rapid autopsy, the focus was kept on the parasinus dura. The presence of dural lymphatics in other dural areas has been documented in animal studies and probably exists in other areas not addressed by this study. The relative number of samples used in this study is small but comparable to other anatomical studies.
Conclusions
AGs connect to the venous circulation via IVCs that stain positively with established lymphatic endothelial cell markers. A better understanding of the morphological and histological features of AGs and IVCs will enhance our study of CSF drainage, clearance of macromolecules from the CNS, and mechanisms of metastasis. The observation that AGs interact with both venous and lymphatic components suggest parallels to lymph nodes in the periphery. Further experiments are necessary to definitively address this outstanding question, but it is fair to theorize that AGs may subserve the role of lymph nodes in the CNS. This finding may have implications for entry and exit of immune cells in the brain and clearance of macromolecules implicated in neurodegenerative diseases.
Acknowledgments
We acknowledge the Dean’s Office of the University of Virginia School of Medicine for partial funding of this study to Dr. Kalani.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Kalani, Yağmurlu, Sokolowski, Shaffrey. Acquisition of data: Yağmurlu, Sokolowski, Soldozy, Norat, Çırak, Tvrdik. Analysis and interpretation of data: Yağmurlu, Sokolowski, Soldozy, Norat, Çırak, Tvrdik. Drafting the article: Kalani, Yağmurlu, Sokolowski, Tvrdik. Critically revising the article: Kalani, Yağmurlu, Shaffrey. Reviewed submitted version of manuscript: Kalani, Yağmurlu. Approved the final version of the manuscript on behalf of all authors: Kalani. Statistical analysis: Kalani, Yağmurlu, Sokolowski, Soldozy. Administrative/technical/material support: Kalani, Yağmurlu, Shaffrey. Study supervision: Kalani, Yağmurlu, Shaffrey.
References
-
1
MascagniP.Vasorum lymphaticorum corporis humani historia et ichnographia.Ex typographia Pazzini Carli;1787.
-
2
LeccoV.Di una probabile modificazione delle fessure linfatiche della parete dei seni venosi della dura madre.Arch Ital Otol Rinol Laringol.1953;64(3.):287–296.
-
3.
LiJ,ZhouJ,ShiY.Scanning electron microscopy of human cerebral meningeal stomata.Ann Anat.1996;178(3.):259–261.
-
4
SandroneS,Moreno-ZambranoD,KipnisJ,vanGijn J.A (delayed) history of the brain lymphatic system.Nat Med.2019;25(4):538–540.
-
5 ↑
LouveauA,SmirnovI,KeyesTJ,et al.Structural and functional features of central nervous system lymphatic vessels.Nature.2015;523(7560):3.3.7–3.41.
-
6 ↑
BowerNI,HoganBM.Brain drains: new insights into brain clearance pathways from lymphatic biology.J Mol Med (Berl).2018;96(5):3.83–3.90.
-
7 ↑
FoxRJ,WaljiAH,MielkeB,et al.Anatomic details of intradural channels in the parasagittal dura: a possible pathway for flow of cerebrospinal fluid.开云体育app官方网站下载入口.1996;3.9(1):84–91.
-
8 ↑
GailloudP,MusterM,KhawN,et al.Anatomic relationship between arachnoid granulations in the transverse sinus and the termination of the vein of Labbé: an angiographic study.神经放射学.2001;43(2):139–143.
-
9 ↑
UptonML,WellerRO.The morphology of cerebrospinal fluid drainage pathways in human arachnoid granulations.J Neurosurg.1985;63(6):867–875.
-
10 ↑
YağmurluK,MooneyMA,AlmeftyKK,et al.An alternative endoscopic anterolateral route to Meckel’s cave: an anatomic feasibility study using a sublabial transmaxillary approach.World Neurosurg.2018;114:134–141.
-
11 ↑
HerzJ,LouveauA,Da MesquitaS,KipnisJ.Morphological and functional analysis of CNS-associated lymphatics.In:Lymphangiogenesis.Humana Press;2018:141–151.
-
12 ↑
WellerRO,DjuandaE,YowHY,CarareRO.大脑的淋巴引流and the pathophysiology of neurological disease.Acta Neuropathol.2009;117(1):1–14.
-
13 ↑
LenckS,RadovanovicI,NicholsonP,et al.Idiopathic intracranial hypertension: the veno glymphatic connections.Neurology.2018;91(11):515–522.
-
14 ↑
CserrHF,KnopfPM.Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view.Immunol Today.1992;13(12):507–512.
-
15 ↑
KidaS,PantazisA,WellerRO.CSF drains directly from the subarachnoid space into nasal lymphatics in the rat.Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol.1993;19(6):480–488.
-
16
DavsonH,WelchK,SegalMB.Physiology and Pathophysiology of the Cerebrospinal Fluid.Churchill Livingstone;1987.
-
17
JohansonCE,DuncanJAIII,KlingePM,et al.Multiplicity of cerebrospinal fluid functions: new challenges in health and disease.Cerebrospinal Fluid Res.2008;5:10.
-
18
AspelundA,AntilaS,ProulxST,et al.A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules.J Exp Med.2015;212(7):991–999.
-
19 ↑
MamourianAC,TowfighiJ.MR of giant arachnoid granulation, a normal variant presenting as a mass within the dural venous sinus.AJNR Am J Neuroradiol.1995;16(4)(suppl):901–904.
-
20 ↑
GrzybowskiDM,HerderickEE,KapoorKG,et al.Human arachnoid granulations Part I: a technique for quantifying area and distribution on the superior surface of the cerebral cortex.Cerebrospinal Fluid Res.2007;4(1):6.
-
21 ↑
AbsintaM,HaSK,NairG,et al.Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI.eLife.2017;6:e29738.
-
22 ↑
JohnstonMG,BoultonM,FlessnerM.Cerebrospinal fluid absorption revisited: do extracranial lymphatics play a role?Neuroscientist.2000;6(2):77–87.