Traditionalmodels of intracranial dynamics are based on pressure-volume relationships in accordance with the Monro-Kellie doctrine.1,2understoo颅内压(ICP)脉冲d as the pressure response to intracranial volume changes associated with the cerebral arterial pulse. Indeed, raising the mean ICP (which lowers intracranial compliance) increases the ICP pulse amplitude, as is expected from pressure-volume relationships.2–4
Paradoxically, however, Foltz and Sklar5,6have shown that the ICP pulse amplitude has a local minimum at normal mean ICP. That is, at lower-than-normal mean ICP, the ICP pulse amplitude increases, just as it does at higher-than-normal ICP. If we make the reasonable assumption that low ICP corresponds to high intracranial compliance, this result is not explained by traditional pressure-volume models, which inherently predict that high compliance associated with low ICP would diminish, not increase, the amplitude of the ICP pulse.
Even more perplexing is the observation that in normal dynamics the ICP pulse precedes the carotid ABP pulse by about one-sixth of the cardiac cycle (Fig. 1A).7This is not explained as a five-sixth cycle lag from the preceding ABP pulse because asystole causes a dropout of the corresponding ICP pulse (Fig. 1B),而不是下面的ICP脉冲。显然,一个ICP pulse that precedes the ABP pulse from which it is presumed to have arisen cannot be explained by our current pressure-volume models.

A:The experimentally measured ICP lead with respect to the arterial pulse in a dog. In normal dynamics, the ICP pulse (dotted line) leads the ABP pulse (solid line) by roughly one-sixth of the cardiac cycle. This is very counterintuitive. From the perspective of traditional pressure-volume models, the ICP pulse is caused by the ABP pulse, and therefore ICP could not precede it. The normal phase lead of the ICP pulse with respect to the ABP pulse implies that traditional pressure-volume models are inadequate to explain the ICP pulse. Reproduced from Wagshul et al.J Neurosurg Pediatr. 2009;3:354-364.7© AANS, published with permission.B:Graphic evidence that the phase lead of the ICP (red line) with respect to the ABP (blue line) is real and not merely a five-sixth cycle lag from the previous ABP pulse. The hypothesis that the phase lead of ICP with respect to ABP is apparent because the ICP pulse is generated by the preceding ABP pulse five-sixths of a cycle earlier is disproven by the presence of asystole in the canine data. In asystole, both the ABP pulse and the slightly preceding ICP pulse disappear, indicating that the leading ICP pulse is causally connected to the ABP pulse that follows it, not the ABP pulse that precedes it. Figure is available in color online only.
Motivated by these paradoxes, recent investigators7–9have characterized the cranium as a frequency-sensitive filter—i.e., a system that selectively transforms the frequency components of the input (ABP pulse) into the output (ICP pulse). Using transfer function analysis, Wagshul et al.7demonstrated that the cranium suppresses the ABP pulse in a stop band around the frequency of the heart rate, which is shown by the attenuation of amplitude in the normal heart rate range in the frequency domain (Fig. 2).This explains Foltz’s and Sklar’s results—the cranium is a steady-state pulsation absorber tuned to the heart rate frequency.5–8

Graphs showing the transfer function of the windkessel in an experimental dog.Left:The amplitude transfer function shows the high-impedance stop band at the heart rate. This represents the suppression of the arterial pulse in the capillaries of the brain parenchyma.Right:The phase transfer function shows a phase transition near the cardiac fundamental, which is characteristic of a pulsation absorber tuned to high-impedance resonance at the heart rate. Theopen circlesmark the fundamental (heart rate) harmonic, as well as the second and third harmonics. The electrical analog of the cerebral windkessel is a tank circuit with capacitance and inductance in parallel. Reproduced from Wagshul et al.J Neurosurg Pediatr. 2009;3:354-364.7© AANS, published with permission.
Three questions arise from the observation that the cranium is a band-stop filter for the suppression of the arterial pulse: 1) How is ICP pulse suppression in the cranium accomplished? 2) What is the physiological purpose of this ICP suppression? 3) What is the relevance of ICP pulse suppression to disorders of intracranial dynamics?
要回答第一个问题,我们模拟了ABP pulse and ICP pulse by using an electrical tank circuit, which is the simplest model of a band-stop filter, and we compared the dynamics of the circuit to physiological data from dogs by using a mathematical technique called autoregressive with exogenous inputs (ARX) modeling. ARX modeling can be used to measure and describe the similarity between two dynamic systems: in this case, the ABP/ICP dynamics of the dogs and the voltage input/output of a simple electrical tank circuit. The model predicts future data points in the ICP waveform as if it were output voltage from a circuit, and we measured its predictive power by how closely this circuit prediction correlated with actual ICP. It is a way of asking: if the cranium worked the same way as the circuit, how similar would the real ICP waveform be to the circuit’s voltage waveform? ARX modeling allowed us to correlate the well-understood dynamics of a simple electrical circuit to the more complex and poorly understood dynamics of the cranium, as well as to gain insight into how the cerebral windkessel works.
To address the second question, we briefly discuss the implications of this insight for our understanding of the intracranial windkessel mechanism, which we suggest may be the purpose for this suppression of the ICP pulse.
探索第三个问题,我们认为是我mplications of windkessel dysfunction for our understanding of hydrocephalus and other disorders of intracranial dynamics.
开云体育世界杯赔率
The Conceptual Basis for the Tank Circuit Model of Cerebral ICP Pulse Suppression
The dynamics of an electrical circuit are analogous to the dynamics of the cranium in many ways. Voltage corresponds to pressure, current to flow and motion, and charge to displacement of fluid and tissue. Inductance corresponds to the size of the pulse, capacitance to parenchymal compliance, and resistance to intracranial damping.
Power is the rate of energy flow, and there is direct current (DC) and alternating current (AC) power in both the circuit and the cranium. In the cranium, DC power is the smooth bulk flow of blood and CSF, whereas AC power is the pulsatile flow of blood and CSF and the pulsatile motion of the brain.
It is noteworthy that while DC and AC power are mixed in arterial and venous blood, DC and AC power within the cranium take distinctly different pathways. DC power drives capillary blood flow (which is nearly pulseless) and AC power drives CSF and brain motion, which is nearly all pulsatile.9
Therefore, cranial arterial inflow and venous outflow is a mix of DC and AC power, yet within the cranium DC power travels almost exclusively in the capillaries and AC power travels almost exclusively in the CSF.
With these observations, we constructed the simplest model of the cerebral windkessel, which is a band-stop filter electrical tank circuit (Fig. 3).We compared this circuit model to ABP and ICP data in 12 normal dogs by using ARX modeling.

A:Circulation flow of the cerebral windkessel and suppression of capillary AC power by the ABP in the capillaries of the cranium are accomplished via cyclic exchange of volume between the intracranial arteries and veins, which are linked by the CSF. This intracranial volume exchange is analogous to the circulation current in a tank circuit, which represents the same dynamics. At every moment throughout the cardiac cycle, the flow of volume in the cerebral windkessel (the extracapillary cranial contents) is equal and opposite to the ABP entering the capillary bed, which cancels the pulse of the arterial inflow to the capillaries and diverts it through the CSF to the intracranial veins.B:This cyclic exchange of volume between the intracranial arteries and veins can be simulated in the tank circuit as the loading and unloading of charge on the "arterial" and "venous" plates of the capacitor in the tank, which are opposite in direction and mutually cancel. This renders capillary blood flow (nearly) pulseless. Figure is available in color online only.
Windkessel Impedance and Windkessel Effectiveness
The band-stop filter effect of the cerebral windkessel is the impedance of the cranium to the arterial pulse. Because the circuit model components are analogous to intracranial fluids and tissues, the impedance of the cranium is the ratio of pressure to flow expressed in complex numbers:

whereZcraniumis the combined impedance of the capillary and CSF path;Rcapis the resistance in the capillaries;RCSFis the damping of pulsatile motion by the brain, the CSF path, and the vasculature;ωis the heart rate expressed in radians;Cis parenchymal compliance;Lis inertance (corresponding to the size of the arterial pulse); andj= −11/2.
The windkessel effect is sensitive to the heart rate (ω) and thus must be tuned.8If we make the reasonable assumption that the normal windkessel represents optimal suppression of the heart rate (seeAppendix 1for details), the complex equation above reduces to a simple equation for what we call windkessel effectiveness: windkessel effectiveness (W) = (tuned)Zcranium=L/(RCSFC).
This equation of windkessel effectiveness is of central importance to our theory of intracranial thermodynamics. An attenuated intracranial pulse (low inertanceL), high CSF path resistanceRCSF, and high parenchymal complianceCimpair windkessel effectiveness. As we will see in theDiscussion, this sensitivity of the windkessel to inertance, resistance, and compliance provides a simple taxonomy of disorders of intracranial dynamics such as hydrocephalus, pseudotumor, and Cushing’s reflex.
Data Collection and Analysis
For quantitative physiological validation of this circuit model, we used data from a series of dogs (18–26 kg) that were previously reported.7Our protocol was approved by our Institutional Animal Care and Use Committee, and the animals were treated humanely in accordance with theGuide for the Care and Use of Laboratory Animalspublished by the National Institutes of Health. Each dog was sedated with thiopental, intubated, and anesthetized with 1.5% isoflurane. A 1-mm-diameter Microtip pressure transducer (SPR-524, Millar Instruments) was inserted into the right common carotid artery by open cutdown and advanced rostral in the artery to just below the skull base and secured. A burr hole was drilled in the right frontal region and the dura mater was opened, and a second Microtip catheter was inserted 1–2 cm into the right frontal parenchyma. Data were collected for approximately 15 minutes in the resting state when ICP was normal, prior to manipulation of ICP. All data analyzed in this paper were obtained from dogs with normal resting ICP.
For a detailed explanation of data processing and ARX modeling, please seeAppendix 2.
Results
Simulation of Circulation Flow in the Tank Circuit
验证坦克circuit model, we simulated circuit flow by using a group of known circuit elements and an input voltage source. Estimation of the model parameters of cerebral ICP pulse suppression based on the physiological values was limited by the lack of experimental data on which to base the parameter values (please seeThe Nature and Limitations of Our Studysection for a full discussion of our selection of parameter values). We set the circuit element values atRcap= 1 Ω,RCSF= 5 Ω,L= 1 H, andC= 0.01 F, and the input voltage was a sinusoidal signal with a DC level of 60 mV. The charge and discharge of the capacitor are shown inFig. 3B, where the variations of the charges on the positive plate and negative plate represent the expansion and relaxation of the arteries and veins. InFig. 4A, we show the phase measured experimentally in dogs; inFig. 4B, we plot the input and output voltages of the model to show phase relationships. The leading ICP phase of the circuit model correlated with the leading ICP phase in the experimental canine model, and this suggested that the counterintuitive phase lead of ICP with respect to ABP in the experimental animals was due to the dynamics inherent to optimal pulsation absorption in a damped system, in accordance with the model proposed by Egnor8(seeDiscussion).

A:The phase lead of ICP (dotted line) with respect to ABP (solid line) in the cerebral windkessel, showing the experimentally measured ICP lead with respect to the arterial pulse in a dog. Reproduced from Wagshul et al.J Neurosurg Pediatr. 2009;3:354-364.7© AANS, published with permission.B:The phase lead of the voltage across the main resistor (dotted line[the ICP proxy]) in the tank circuit model and the voltage across the source (solid line[the ABP proxy]). The tank circuit model naturally produces the phase lead of ICP to ABP observed in dogs. Figure is available in color online only.
Model Evaluation
ICP Prediction by the ARX Model
We evaluated the time series model by calculating the relative errors of ICP prediction. Ten-second windows were used to analyze the segments of processed ABP and ICP. We estimated the coefficientsai, i= 1, 2, andbj, j= 0, 1, 2, by fitting segments in the model and predicting ICP at the next time instant according to the coefficients.Table 1summarizes the average relative prediction error for each canine data set.
Average relative error of ICP prediction based on the ARX model
| Data Set | Average Relative Error (%) | Data Set | Average Relative Error (%) |
|---|---|---|---|
| No. 1 | 0.48 | No. 7 | 2.75 |
| No. 2 | 1.81 | No. 8 | 0.50 |
| No. 3 | 0.16 | No. 9 | 2.10 |
| No. 4 | 0.48 | No. 10 | 0.67 |
| No. 5 | 0.94 | No. 11 | 1.31 |
| No. 6 | 1.14 | No. 12 | 0.75 |
Circuit Element Estimation and Transfer Function Comparison
Given the estimated coefficients of the ARX model, we correlated the proposed circuit model with the ARX model and estimated the circuit elements by using the method shown above. The 10-second segments of ABP and ICP from the canine data sets were selected for testing. Among 12 dogs, we chose a total of 16 segments from 4 dogs and summarized their parameters, estimating the circuit elements inTable 2. InFig. 5, we compare the gain of the transfer function from the fast Fourier transform (FFT), ARX, and circuit models according to the estimated circuit elements. The figure suggests similarity between the transfer functions of the model and the actual data from the dogs.
The parameters of the canine data and corresponding estimation results of the tank circuit
| Data Set | Segments (sec) | Heart Rate (Hz) | Mean ICP (mm Hg) | Rcap | RCSF | L | C |
|---|---|---|---|---|---|---|---|
| Dog 3 | 640–650 | 0.60 | 23.71 | 0.6959 | 6.4382 | 5.1452 | 0.0119 |
| Dog 3 | 690–700 | 0.76 | 23.39 | 1.0012 | 5.3729 | 3.7022 | 0.0102 |
| Dog 3 | 740–750 | 0.91 | 22.63 | 0.6659 | 7.9382 | 3.0083 | 0.0090 |
| Dog 3 | 950–960 | 0.61 | 21.63 | 0.3049 | 5.7786 | 2.6780 | 0.0250 |
| Dog 5 | 260–270 | 0.89 | 23.07 | 3.0743 | 5.2010 | 2.2801 | 0.0138 |
| Dog 5 | 410–420 | 0.89 | 22.70 | 0.5493 | 1.6838 | 0.5819 | 0.0890 |
| Dog 5 | 500–510 | 0.69 | 22.66 | 1.4190 | 2.6838 | 1.2882 | 0.0322 |
| Dog 5 | 570–580 | 0.69 | 22.77 | 1.0802 | 2.3482 | 1.3001 | 0.0621 |
| Dog 10 | 130–140 | 0.50 | 6.5 | 0.9010 | 8.0352 | 4.0335 | 0.0195 |
| Dog 10 | 180–190 | 0.50 | 5.9 | 0.5624 | 6.0352 | 5.6320 | 0.0136 |
| Dog 10 | 250–260 | 0.63 | 5.5 | 0.5630 | 6.1832 | 3.8210 | 0.0199 |
| Dog 10 | 350–360 | 0.87 | 4.9 | 0.8218 | 9.3989 | 2.2089 | 0.0129 |
| Dog 12 | 230–240 | 0.98 | 5.6 | 0.3760 | 6.7054 | 5.3270 | 0.0114 |
| Dog 12 | 260–270 | 0.98 | 6.2 | 0.5102 | 6.4355 | 3.4145 | 0.0088 |
| Dog 12 | 310–320 | 0.98 | 6.9 | 0.7790 | 8.1562 | 4.5355 | 0.0060 |
| Dog 12 | 360–370 | 0.98 | 8.6 | 0.9495 | 8.0346 | 4.7178 | 0.0051 |
Four 10-second segments from dogs 3, 5, 10, and 12 were selected for testing. Dogs 3 and 5 had relatively large mean ICP values, and dogs 10 and 12 had small mean ICP values. Both ICP values represent normal baseline dynamics in these animals. InFig. 6, we plotted the transfer functions of the circuit according to the results of dog 12, shown in this table.

Comparison of the transfer functions of the windkessel model and canine data. Time domain data are shown in the 2 graphs in theupper panel, and frequency domain data are shown in the 4 graphs in thelower panel. The transfer function from the output ICP to input ABP of the canine data can be obtained directly by computing the FFT. According to the model, the transfer functions of the ARX and circuit models should capture the salient features of the transfer function from the canine data. The transfer function of the circuit is approximately equivalent to the ARX model obtained from the dog near the heart rate frequency. In this figure, we show the results of four examples with normal mean ICP. Figure is available in color online only.
Discussion
The Nature and Limitations of Our Study
Our study shows a close correlation between the pulsatile intracranial dynamics of dogs and the pulsatile dynamics of the electrical tank circuit, which suggests similar underlying mechanisms of pulse suppression.
Our research entailed several distinct experiments. In the first experiment (simulation of the circuit model), we selected the parameters of the circuit elements on the basis of the properties observed in the cranium (e.g., suppression of the ABP pulse and the phase lead of the ICP pulse). The results show that the proposed circuit model can produce these dynamics.
A limitation of the first experiment was that selection of the circuit model parameters was limited by the lack of measured values of these parameters in the cranium. The damping, compliance, and inertance relevant to our study are parameters specific to the radial micromotion of the capillary walls in the brain parenchyma, which has never been measured.
Given these constraints, we chose the circuit parameters of resistance, capacitance, and inductance in our first experiment based on three considerations. 1) We noted that there is conceptual and physical continuity between windkessel dynamics in the aorta as described by Frank10and windkessel dynamics in the cranium. The pulsatile storage and release of blood in the aorta were contiguous with the pulsatile storage of blood in the intracranial arteries, so we set the parameters in the simulation to correlate with values of resistance, capacitance, and inertance measured in the aorta by Stergiopulos et al.112) We set the ratio betweenRcapandRCSFin the simulation to correspond to our empirically measured phase lead of ICP to ABP at the frequency of the heart rate. 3) We set the input and output voltages in the simulation to reflect the relative magnitudes of ABP and ICP, respectively.
In the second experiment, we verified the accuracy of the prediction of the ARX model of the canine data (unrelated to the circuit model). The ARX model is a linear representation of a dynamic system in discrete time.
In the third experiment, we estimated the circuit model elements by linking the ARX model and the circuit model with data from dogs. By comparing the transfer functions from the FFT, the ARX model, and the circuit model, we found that the circuit model with estimated parameters closely matched both the FFT and the ARX model of the canine data.
Using our method, we found a circuit model with estimated parameters that simulated the cerebral windkessel in dogs.
Correspondence Between the Model of ICP Pulse Suppression and the Cranium
ARX modeling confirmed close similarity between the circuit model and the dynamics of the cranium, with a relative error of only 0.16% to 2.75% (Table 1).This indicates that there is a mechanical basis for pulse suppression shared by the cranium and the circuit. Several questions arise: how is ICP pulse suppression in the cranium accomplished, what is the physiological purpose of this ICP suppression, and what is the relevance of ICP pulse suppression to disorders of intracranial dynamics?
How Is ICP Pulse Suppression in the Cranium Accomplished?
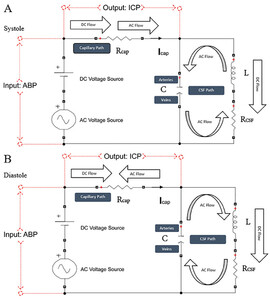
If we consider the AC cycle in the electrical circuit as systole and diastole and define AC flow in the main (capillary) resistor away from the source as cranial and that toward the source as caudal, in systole the AC source current flows cranially while the circulation current from the tank flows caudally. In diastole, the source current flows caudally while the circulation current from the tank flows cranially. The AC source current and the circulation current are continuously equal in magnitude and opposite in direction and meet in the main (capillary) path, where the currents cancel each other (Fig. 6).取消频率selective-only ACsource current at the antiresonant frequency of the circulation current is cancelled. Note that DC power passes through the main (capillary) path unimpeded by the circulation current because DC flow is insensitive to frequency and the reactance of the tank circuit only opposes AC (i.e., accelerated) flow. In other words, the tank circuit selectively passes DC power and impedes AC power.

Our schematic windkessel circuit model in systole and diastole. The tank circuit has a DC and AC source (corresponding in the cranium to mean arterial pressure and pulse pressure, respectively); a series resistorRcap(corresponding to the capillary path); and a parallel resistance, inductor, capacitor (RLC) circuit (corresponding to the CSF, interstitial fluid, brain parenchyma, and intracranial arteries and veins, which for brevity we call the CSF path). Voltage represents pressure, current represents flow, and charge represents volume. Voltage across the source is a proxy for ABP, and voltage across the capillary resistor is a proxy for ICP. Capacitance (C) represents the compliance of the extracapillary (parenchymal and CSF) path and corresponds primarily to the compliance of the intracranial veins. Inductance (L) represents intracranial inertance, which corresponds to the inertance of the extracapillary cranial contents.RCSFrepresents resistance to the CSF pulse in the CSF spaces, structural damping of the vascular walls and brain parenchyma, and resistance to compression/expansion of the cerebral arteries and veins.RCSFis effectively a venous pump.Rcapcorresponds to intracapillary resistance to longitudinal blood flow, andRCSFis the net resistance in the extracapillary space to radial expansion and relaxation of capillary walls. Note thatRCSFdoes not represent the resistance to CSF absorption. The DC power that drives CSF formation and absorption is several orders of magnitude smaller than AC power and is represented in this model as a miniscule component of DC power. The model clarifies the dynamics of the cerebral windkessel. Suppression of the AC current from the capillary path is accomplished by cyclic loading and unloading of the "arterial" and "venous" capacitor plates, which continuously oppose and block the cyclic current from the AC arterial source. This circulation current is the motion of the brain and CSF in the cardiac cycle; in systole, the brain expands and moves caudally, and in diastole it relaxes and rebounds cranially.12The AC power is shunted through the CSF to the veins by CSF-coupled cyclic arterial expansion linked to venous compression in systole and arterial relaxation linked to venous re-expansion in diastole. This accomplishes the cerebral windkessel. Figure is available in color online only.
The close correspondence between the voltage in the model and the ICP pulse suggests that the ICP pulse is suppressed by the analogous motion of the intracranial contents that continuously oppose the motion of arterial blood entering the cranium. Such motion was observed by Greitz using flow MRI.12In systole, the caudal motion of the brain opposes the cranial motion of the arterial pulse entering at the skull base. The systolic centrifugal motion of the brain parenchyma opposes the centripetal inflow of the arterial pulse in the perforating cortical arteries, and radial inward compression by the expanding brain parenchyma opposes radial outward expansion of the capillary walls.8In diastole, cranial rebound of the brain opposes diastolic caudal regress of the arterial pulse at the skull base. Diastolic centripetal motion of the cortex opposes diastolic centrifugal regress of the arterial pulse in the perforating arteries, and centrifugal relaxation of the brain parenchyma opposes centripetal relaxation of the capillary walls.8,12This continuous balance of flow in the cranium is analogous to the circulation current in the tank circuit.
We propose that this intracranial circulation current is the physiological basis for the cerebral windkessel.
ICP脉冲一口的生理目的是什么pression?
这是非凡的ICP脉冲抑制exists—the dynamics of the cranium certainly could be such that the ABP-ICP transfer is augmented, suppressed, unaffected, or even variable with time. The specific band-stop filter dynamics inherent to the cerebral windkessel imply a physiological purpose, and we believe that this purpose is to protect the capillary bed from pulsatility. Focusing on the thermodynamics of cerebral blood flow and ICP, both DC and AC power enter the cranium via arterial perfusion. DC power (smooth flow) passes through the capillaries unimpeded, but AC power is impeded in the vasculature and passes instead through the CSF spaces to the veins. This provides smooth effective capillary perfusion.
Proper "tuning" of this band-stop windkessel effect in the cranium implies physiological regulation of several parameters—intracranial inertance, compliance, damping, and heart rate—although the physiological mechanism by which this windkessel tuning is accomplished is not understood.
Why Does the ICP Pulse Precede the ABP Pulse?
The windkessel model offers an explanation for the perplexing phase lead of ICP with respect to ABP that we noted in theIntroduction. The cranium is a pulsatile system in the steady state, and as such, the individual frequency components of the ICP can lead or lag the corresponding frequency components of the ABP in accordance with the inertance, compliance, and resistance of the cranium. In a damped steady-state oscillator such as the cranium, pulse suppression is enhanced by a somewhat "heavy" pulse (i.e., an inertial bias in the reactance) because normal damping in the cranium creates frictional loss that degrades pulse suppression. An increase in the mass of the pulse helps overcome this frictional loss, and this inertial bias creates a leading ICP phase. The phase lead of ICP to ABP represents optimal windkessel function in a damped cranium. This is discussed in more detail by Egnor.8
What Is the Relevance of ICP Pulse Suppression to Disorders of Intracranial Dynamics?
On flow MRI, normal CSF pulsations are quite brisk, whereas CSF bulk flow is barely detectable.12The measured ratio of the AC/DC energy of intracranial CSF flow is approximately 100,000:1—i.e., nearly all the energy of CSF motion is in the AC component.9This new perspective on windkessel energy dynamics points to explanations for several common disorders of intracranial dynamics that are quite different from the traditional understanding.
In traditional pressure-volume models, hydrocephalus is understood as high resistance to the absorption of CSF, and ventriculomegaly is due to the backup of CSF. In windkessel theory, hydrocephalus is understood as high impedance to AC power in the CSF pathway, and ventriculomegaly is adaptive—it tends to restore CSF path volume and thereby reduce impedance to AC power.
To understand this in more detail, consider the following equation, which expresses the effectiveness of the tuned cerebral windkessel:L/(RCSFC) = windkessel effectiveness. Here,Lis inertance (the size of the pulse in the CSF path),RCSFis the resistance to AC power in the CSF path, andCis compliance in the CSF path. High windkessel impedance in the CSF path can happen in three ways: increasedRCSF, increasedC, or decreasedL. Although pathological CSF path impedance inherently involves some abnormalities in all three parameters, we focused our considerations on the primary abnormalities. This points to a taxonomy of hydrocephalus based on windkessel impedance.
Obstructive Hydrocephalus: High Windkessel Impedance due to High Resistance to AC Power in the CSF Path
Increased resistance to AC power in the CSF pathways (e.g., from subarachnoid or ventricular obstruction) is a loss of CSF path volume to AC power and impairs windkessel effectiveness:L/(↑RCSFC) = ↓windkessel effectiveness in obstructive hydrocephalus.
This loss of CSF path volume can have dire physiological consequences, with "gluing" of the scarred CSF path and rerouting of AC power to the capillary circulation with consequent capillary disruption, edema, and herniation. Restoration of the volume of the CSF path (i.e., via ventriculomegaly) may partially or completely ameliorate this impairment.
In windkessel theory, ventriculomegaly is an adaptation to hydrocephalus—a restoration of CSF volume for the transmission of AC power—not a passive backup of CSF due to malabsorption. The physiological causes of adaptive ventriculomegaly, and whether it is a passive or active process, remain obscure.
NPH: High Windkessel Impedance due to Low Inertance and High Compliance in the CSF Path
The combination of low inertance and high compliance to AC power in the CSF pathways (i.e., due to arteriosclerosis which blunts the CSF pulse in the subarachnoid space,12as well as age-related brain atrophy and softening which increase the compliance of subarachnoid CSF pathways) can impair windkessel effectiveness:L↓/(RCSFC↑) = ↓windkessel effectiveness in normal pressure hydrocephalus (NPH).
This inertance and compliance-related windkessel impairment in NPH causes adaptive ventriculomegaly, as in obstructive hydrocephalus, but compliance is high and the windkessel is not "glued" by high resistance and is still able to function, albeit with a higher-than-normal ventricular pulse amplitude and with increased stress on the periventricular white matter tracts innervating the legs and bladder.
The different physiological effects of high-resistance windkessel impairment (obstructive hydrocephalus) and of low inertance–high compliance windkessel impairment (NPH) would account for the notable clinical differences between these disorders.
CSF Diversion
Windkessel theory suggests a new understanding of CSF diversion. CSF diversion drains energy contained in CSF, which lowers intracranial energy density (i.e., ICP). Shunting reduces ventricular size because the shunt is an additional path for AC power and thereby lowers resistance in the CSF path, which restores the windkessel and obviates the need for adaptive ventriculomegaly. A shunt, in essence, is an accessory windkessel:L/(↓RCSFC) = ↑windkessel effectiveness.
Low-Pressure Hydrocephalus: High Windkessel Impedance due to High Resistance and High Compliance in the CSF Path
The combination of high resistance and high compliance (i.e., low brain turgor) in the CSF path can cause windkessel impairment in a chronically shunted patient. This hydrocephalus syndrome would present with massive ventriculomegaly but low ICP due to the pathologically high intracranial compliance, orL/(↑RCSFC↑) = ↓↓windkessel effectiveness in low-pressure hydrocephalus.
It is noteworthy that the two methods for treating low-pressure hydrocephalus—CSF siphoning and neck wrapping13—specifically reverse the high resistance and high compliance that are characteristic of this kind of windkessel impairment, orL/(↓RCSFC) = ↑windkessel effectiveness due to CSF siphoning orL/(RCSFC↓) = ↑windkessel effectiveness due to neck wrapping.
Pseudotumor Cerebri
The absence of ventricular dilation in pseudotumor cerebri is readily explained by the observation that pseudotumor is an impairment of the flow of DC power in the capillary bed (due to cerebrovenous hypertension), without impairment of AC power flow in the CSF path (the CSF path is normal). Adaptive ventriculomegaly is of no physiological benefit in pseudotumor and does not occur.14Shunting is effective in pseudotumor because shunts drain power and thereby lower ICP.
Cushing’s Reflex
Windkessel theory offers insights into the physiological rationale for Cushing’s reflex. The purpose of the windkessel is to maintain normal DC power of capillary perfusion and to divert AC power away from the capillaries through the CSF to the cerebral veins. In the setting of markedly increased resistance to AC power in the CSF pathways due to decompensated hydrocephalus or severe brain swelling with CSF space obliteration, the cerebral windkessel is dangerously impaired. From this perspective, arterial hypertension and bradycardia are adaptations that protect windkessel function via 1) maintenance of capillary DC perfusion (arterial hypertension); 2) reduction of net AC power entering the cranium (bradycardia); and 3) augmentation of intracranial inertanceL(increase of the strength of the arterial pulse). Cushing’s reflex can be understood as an accessory windkessel in extremis.
Conclusions
There are serious problems with the pressure-volume models of intracranial dynamics. Evidence shows that the cranium is a band-stop filter for the heartbeat—i.e., the cranium is a windkessel—and the cranial windkessel is very similar quantitively to a simple electrical tank circuit. The dynamics underlying this similarity point to a mechanism by which the cranial windkessel works—the buffering of the arterial pulse in the brain capillaries is accomplished by the rhythmic motion of the cranial contents during the cardiac cycle that opposes the arterial pulse. This intracranial windkessel depends on the simultaneous coupling of arterial expansion and venous compression via CSF hydraulics. The thermodynamics underlying the windkessel and the mathematics describing it point to a new understanding of the pathophysiology and treatment of hydrocephalus and other disorders of intracranial dynamics.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Egnor, Yang, Mani, Fiore, Djurić. Acquisition of data: Egnor, Yang. Analysis and interpretation of data: Egnor, Yang, Mani, Djurić. Drafting the article: Egnor, Yang, Mani, Djurić. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Egnor. Statistical analysis: Yang, Djurić. Administrative/technical/material support: Egnor, Mani, Fiore. Study supervision: Egnor, Djurić.
Supplemental Information
Online-Only Content
Supplemental material is available with the online version of the article.
Appendices 1 and 2.//www.prize-show.com/doi/suppl/10.3171/2023.1.PEDS22372.
References
-
1 ↑
WilsonMH.Monro-Kellie 2.0: the dynamic vascular and venous pathophysiological components of intracranial pressure.J Cereb Blood Flow Metab.2016;36(8):1338–1350.
-
2 ↑
UrsinoM,GiulioniM,LodiCA.Relationships among cerebral perfusion pressure, autoregulation, and transcranial Doppler waveform: a modeling study.J Neurosurg.1998;89(2):255–266.
-
3
UrsinoM,LodiCA.A simple mathematical model of the interaction between intracranial pressure and cerebral hemodynamics.J Appl Physiol (1985).1997;82(4):1256–1269.
-
4 ↑
ZhengY,MayhewJ.A time-invariant visco-elastic windkessel model relating blood flow and blood volume.2009;47(4):1371–1380.
-
5 ↑
FoltzEL.Hydrocephalus and CSF pulsatility: clinical and laboratory studies. In:ShapiroK,MarmarouA, eds.Hydrocephalus.Raven;1984;337-362.
-
6 ↑
SklarFH,ElashviliI.The pressure-volume function of brain elasticity. Physiological considerations and clinical applications.J Neurosurg.1977;47(5):670–679.
-
7 ↑
WagshulME,KellyEJ,YuHJ,GarlickB,ZimmermanT,EgnorMR.Resonant and notch behavior in intracranial pressure dynamics.J Neurosurg Pediatr.2009;3(5):354–364.
-
9 ↑
EgnorM,RosielloA,ZhengL.A model of intracranial pulsations.Pediatr Neurosurg.2001;35(6):284–298.
-
10 ↑
FrankO.Die Grundform des arterielen Pulses erste Abhandlung: mathematische Analyse.Z Biol (Münch).1899;37:483–526.
-
11 ↑
StergiopulosN,WesterhofBE,WesterhofN.Total arterial inertance as the fourth element of the windkessel model.Am J Physiol.1999;276(1):H81–H88.
-
12 ↑
GreitzD.Cerebrospinal fluid circulation and associated intracranial dynamics. A radiologic investigation using MR imaging and radionuclide cisternography.Acta Radiol Suppl.1993;386(1):23.
-
13 ↑
FilippidisAS,KalaniMY,NakajiP,RekateHL.Negative-pressure and low-pressure hydrocephalus: the role of cerebrospinal fluid leaks resulting from surgical approaches to the cranial base.J Neurosurg.2011;115(5):1031–1037.
-
14 ↑
WangZ,YangL,DjurićPM,EgnorMR.Why don’t ventricles dilate in pseudotumor cerebri? A circuit model of the cerebral windkessel.J Neurosurg Pediatr.2022;29(6):719–726.