Anabdominal pseudocyst (APC) is a rare distal catheter site–specific failure in children with ventriculoperitoneal shunts.1The specific cause of APC is not well understood.2Children often present with signs of shunt failure or abdominal complaints but rarely with infection.3The typical surgical management strategy for APC involves complete removal of all shunt components or externalization of distal peritoneal tubing, usually with antibiotic therapy, followed by shunt replacement. At the time of shunt replacement, the clinician must decide whether to place the distal catheter within the peritoneal cavity or in a nonperitoneal location, but there are no clear guidelines for initial surgical management or the optimal location for the shunt terminus. It is unknown whether reinsertion into the peritoneal cavity predisposes to future infection or revision. The risk factors that may predispose patients to APC formation are also not fully known. Gmeiner et al.3found no association between APC formation and age at first surgical procedure, age at first peritoneal catheter, type of first surgical procedure, or etiology of hydrocephalus, but their study was small and probably underpowered (n = 14). Others have found that myelomeningocele etiology,4multiple shunt revisions,2,5and shunt infection6were associated with higher risk of APC formation.
管理的大规模的研究和成果of children diagnosed with an APC has not been performed. In this study, we investigated the management and outcomes of APC in children with shunted hydrocephalus who were treated at centers in the Hydrocephalus Clinical Research Network (HCRN). We hypothesized that management of APC varies among HCRN centers but that there is no difference in future shunt failure rates or causes based on APC management and ultimate reimplantation (abdominal) or implantation (nonperitoneal) site.
开云体育世界杯赔率
Patient Identification
从潜在HCRN核心数据提取Data Project (i.e., Registry) for all children treated for an APC between April 2008 and May 2022 at 14 HCRN centers (Children’s of Alabama; Primary Children’s Hospital; Seattle Children’s Hospital; Children’s Hospital of Pittsburgh; St. Louis Children’s Hospital; Texas Children’s Hospital; Hospital for Sick Children; Monroe Carell Jr. Children’s Hospital at Vanderbilt; British Columbia Children’s Hospital; Alberta Children’s Hospital; Children’s Hospital of Los Angeles; Children’s Hospital Colorado; Nationwide Children’s Hospital; Johns Hopkins Children’s Center). The Registry tracks all hydrocephalus-related shunt surgeries at each center starting from the date of joining the HCRN to the present. Institutional review board approval with waiver of individual patient consent was obtained from each clinical site as well as the data coordinating center.7
Pediatric patients (≤ 18 years of age) undergoing treatment for an APC at any participating HCRN site who had at least 6 months of follow-up after shunt reimplantation in the abdomen or implantation in a nonperitoneal site were included. Cases were identified by the "abdominal pseudocyst" data field on the HCRN Registry infection form. A pseudocyst was recorded when there was a loculated fluid collection in the abdomen containing the peritoneal catheter, with abdominal distention and/or displacement of peritoneal contents, even if organisms were not recovered from the CSF, given that this is specifically designated as an infection per HCRN protocols.
Data Collection
Clinical factors collected included age (at diagnosis of pseudocyst and at initial shunt surgery), etiology of hydrocephalus, time from previous surgery, presence of a gastrostomy tube, number of previous shunt surgeries, previous endoscopic shunt procedure, and number of complex chronic conditions. Factors collected from the shunt surgery immediately before APC diagnosis included the following: previous shunt surgery type (revision/reimplant after infection/new shunt); revision type (proximal, distal, both, valve only); proximal entry site (anterior—near the coronal suture, posterior—near the lambdoid suture); time from preceding shunt surgery; shunt surgery within 12 weeks of APC diagnosis; and peritoneal entry method (minilaparotomy, laparoscopic, trocar, previous tract used without other methods). APC treatment characteristics included initial management strategy (shunt revised, shunt removed/external ventricular drain [EVD] inserted, shunt externalized); duration of EVD/externalization; shunt terminus (peritoneal, nonperitoneal); complexity of the shunt system reimplanted after APC treatment; valve type; and peritoneal entry method on reimplantation. Microbiology information included CSF Gram stain/culture results, APC culture results, white blood cell count, red blood cell count, protein level, CSF culture results before shunt reimplantation, and length of time positive cultures were held for growth.
Outcomes
The primary outcome for the study was shunt failure (any hydrocephalus-related surgical procedure) after APC management at 6-, 12-, and 24-month time points. The secondary outcomes included type of shunt failure (revision/infection), hydrocephalus-related perioperative complications before hospital discharge, and the total number of hydrocephalus procedures within 6-, 12-, and 24-month intervals.
Statistical Analysis
描述性统计是用来总结patient demographics and outcome measures and are reported as counts and percentages for categorical variables and as the mean, median, and first and third quartile (IQR) for continuous variables. Associations among continuous variables were assessed using a Wilcoxon rank-sum test. All categorical variables were compared using a Fisher’s exact test. Time-dependent events were evaluated with Kaplan-Meier curves and compared with a log-rank test. Risk factors for failure were assessed with a Cox proportional hazards model. Variables included in the model were those with p < 0.2 on univariable analysis or those identified as clinically important based on prior studies. The proportional hazards assumption was verified as plausible. All analyses were conducted using SAS version 9.4, and significance levels were set at p < 0.05.
Results
Patient Population
共有155名患者的APC标识符d in our database of 14,080 shunt procedures in which the distal catheter was placed in the peritoneal cavity, which includes 763 shunt infections (from which the pseudocyst cohort is derived). Pseudocysts occurred with a prevalence of 1.1% among all shunt surgeries terminating in the abdomen and comprise 20.3% of those shunt infections. Eight children with APC underwent shunt removal because of infection and were discharged without replacement (2 underwent endoscopic third ventriculostomy [ETV], 6 did not require shunt replacement). Of the remaining 147 patients, 6 children underwent revision without removal/externalization and were removed from the primary analysis (all shunts failed within 30 days). Therefore, 141 children were included in the current analysis, all of whom experienced an initial occurrence of an APC (Fig. 1). After treatment, 70 shunts were reimplanted into the abdominal cavity and 71 shunts were implanted at a nonperitoneal site.

CONSORT diagram demonstrating population for study.
Baseline Demographics
Overall, 57.4% of children (81/141) in the study were male (Table 1). Most children were white (91, 64.5%) and not Hispanic/Latino (116, 82.3%). The median age at initial shunt procedure was 0.4 years (IQR 0.3, 1.3 years) and the median age at reimplant surgery after APC was 9.5 years (IQR 4.6, 14.2 years). The most common etiology of hydrocephalus was intraventricular hemorrhage of prematurity (65, 46.1%). Complex chronic conditions were present in 87 children (61.7%), and 23 (16.3%) children had a gastrostomy tube. Children in the cohort had undergone a median of 2 shunt surgeries before APC diagnosis.
Comparison of patient and treatment factors between peritoneal reimplant and nonperitoneal implant groups*
| Variable | Full Cohort, n = 141 | Shunt Reimplanted in Abdominal Cavity, n = 70 | Shunt Implanted in Nonperitoneal Site, n = 71 | p Value |
|---|---|---|---|---|
| Clinical presentation | ||||
| Male sex | 81 (57.4%) | 37 (52.9%) | 44 (62.0%) | 0.309† |
| Age at initial shunt procedure, yrs | 0.4 [0.3, 1.3] | 0.4 [0.2, 2.7] | 0.5 [0.3, 0.9] | 0.801‡ |
| Age at reimplant surgery, yrs | 9.5 [4.6, 14.2] | 8.4 [3.5, 13.4] | 10.6 [6.2, 14.9] | 0.018‡ |
| Cause of hydrocephalus | 0.952† | |||
| pIVH | 65 (46.1%) | 34 (48.6%) | 31 (43.7%) | |
| Myelomeningocele | 30 (21.3%) | 14 (20.0%) | 16 (22.5%) | |
| Aqueductal stenosis | 8 (5.7%) | 4 (5.7%) | 4 (5.6%) | |
| Other etiology | 38 (27.0%) | 18 (25.7%) | 20 (28.2%) | |
| Complex chronic conditions | 0.173§ | |||
| 0 | 54 (38.3%) | 30 (42.9%) | 24 (33.8%) | |
| 1 | 57 (40.4%) | 28 (40.0%) | 29 (40.8%) | |
| ≥2 | 30 (21.3%) | 12 (17.1%) | 18 (25.4%) | |
| Gastrostomy | 23 (16.3%) | 12 (17.1%) | 11 (15.5%) | 0.823† |
| No. of previous shunt surgeries* | 2 [1, 3] | 2 [1, 3] | 2 [1, 5] | 0.483‡ |
| Most recent surgery prior to APC Dx* | ||||
| Time from previous shunt surgery to APC Dx, mos* | 3.8 [1.5, 27.3] | 3.7 [1.5, 25.2] | 3.9 [1.5, 34.3] | 0.989‡ |
| Type of shunt surgery | 0.647† | |||
| Revision | 61/86 (70.9%) | 37/51 (72.5%) | 24/35 (68.6%) | |
| Infection | 9/86 (10.5%) | 4/51 (7.8%) | 5/35 (14.3%) | |
| New shunt | 16/86 (18.6%) | 10/51 (19.6%) | 6/35 (17.1%) | |
| Type of revision | 0.002† | |||
| Proximal | 29/61 (47.5%) | 23/37 (62.2%) | 6/24 (25.0%) | |
| Distal | 15/61 (24.6%) | 4/37 (10.8%) | 11/24 (45.8%) | |
| Both | 13/61 (21.3%) | 9/37 (24.3%) | 4/24 (16.7%) | |
| Valve revision only | 4/61 (6.6%) | 1/37 (2.7%) | 3/24 (12.5%) | |
| Shunt surgery w/in previous 12 wks | 37/86 (43.0%) | 21/51 (41.2%) | 16/35 (45.7%) | 0.8248† |
| Culture results | ||||
| APC culture positive | 20/141 (14.2%) | 9/70 (12.9%) | 11/71 (15.5%) | 0.810† |
| CSF culture positive | 22/141 (15.6%) | 11/70 (15.7%) | 11/71 (15.5%) | >0.99† |
| APC or CSF culture positive | 25/141 (17.7%) | 12/70 (17.1%) | 13/71 (18.3%) | >0.99† |
| Tx of pseudocyst | ||||
| Surgery | 0.006† | |||
| Total removal w/ EVD | 42/141 (29.8%) | 29/70 (41.4%) | 13/71 (18.3%) | |
| Externalization | 90/141 (63.8%) | 39/70 (55.7%) | 51/71 (71.8%) | |
| Failed externalization w/ subsequent removal | 9/141 (6.4%) | 2/70 (2.9%) | 7/71 (9.9%) | |
| Duration of EVD/externalization, days | 10 [7, 14] | 9.5 [7, 14] | 11 [7, 14] | 0.836‡ |
Dx = diagnosis; pIVH = intraventricular hemorrhage of prematurity; Tx = treatment.
Values are expressed as number (%) or median [Q1, Q3]. Boldface type indicates statistical significance.
Information on all previous procedures is unknown for children whose primary procedure is not in the registry.
Fisher’s exact test.
Wilcoxon rank-sum test.
Cochran-Armitage trend test.
Previous Shunt Surgery Before APC Diagnosis
Some patients (n = 55) had shunt surgery before entering the HCRN Registry, so information about their shunt surgery before APC diagnosis was unavailable. Among the 86 children with complete information about prior shunt surgeries, the median time from previous shunt operation to APC formation was 3.8 months (IQR 1.5, 27.3 months), and the most common preceding operation was shunt revision (61/86, 70.9%). Revision surgery was proximal in 29 children (47.5%) and distal in 15 (24.6%). Thirty-seven children (43.0%) had undergone shunt surgery within the previous 12 weeks. Among the 20 patients for whom it was recorded, an antibiotic-impregnated catheter was used in 17.
Culture Results
Pseudocyst fluid and CSF were sent for culture in all children. In 25 (17.7%) children, there was a positive fluid culture from either the pseudocyst or the CSF: APC cultures were positive in 20 (14.2%) and CSF cultures in 22 (15.6%) (Table 1). The median number of days from fluid sampling to positive culture result was 4 (IQR 3, 6 days), with positive cultures reported a half-day earlier in those catheters implanted at a nonperitoneal site (4 days [IQR 3.5, 11.5 days] vs 4.5 days [IQR 3, 6 days], p = 0.717). The number of days that cultures were monitored before being reported as negative was unknown.
Gram-positive organisms were the most common type on both APC (45%) and CSF (59%) cultures (Table 2). Among all children with positive cultures, gram-positive–only cultures occurred most frequently (Staphylococcusspecies). Three patients had gram-positive organisms on CSF cultures and gram-negative growth on APC cultures. One child’s cultures grew acid-fast bacilli, and another child’s cultures showed fungal growth with an associated gram-positive organism.
Culture results including the types and names of organisms identified in each patient
| Type of Organism | APC Organism(s) | 脑脊液生物(s) |
|---|---|---|
| Gram-positive only | ||
| Staphyloccus caprae, S. capitis, S. hominis | Isolated mixed positive bacteria,S. hominis, S. warneri | |
| S. aureus | S. aureus | |
| S. epidermidis/CoNS | S. epidermidis/CoNS | |
| S. aureus | S. aureus | |
| S. aureus | S. aureus, Cutibacterium acnes | |
| S. epidermidis/CoNS,C. acnes | S. epidermidis/CoNS | |
| S. epidermidis/CoNS | S. epidermidis/CoNS | |
| S. caprae, S. warneri | S. epidermidis/CoNS,S. caprae, S. warneri | |
| S. capitis | S. capitis, S. capitis | |
| C. acnes | ||
| S. aureus, S. epidermidis | ||
| S. epidermidisin broth | ||
| C. acnes, S. capitis | ||
| Fungal, gram-positive | Candida albicans, Lactobacillusspecies | |
| Gram-negative only | ||
| Klebsiella pneumoniae | K. pneumoniae | |
| k . oxytoca | k . oxytoca | |
| Prevotellaspecies | ||
| Pseudomonas aeruginosa | P. aeruginosa | |
| k . oxytoca, Escherichia coli, Enterococcus faecalis, Enterobacter cloacae | ||
| E. cloacae | E. cloacae | |
| Haemophilus influenzae | ||
| Gram-negative/gram-positive | ||
| OccasionalNeisseria gonorrhoeae | OccasionalNeisseria gonorrhoeae, C. acnesin broth | |
| P. aeruginosa | P. aeruginosa, E. coli, staphylococcus in broth | |
| Serratia marcescens | S. aureus | |
| Acid-fast bacilli | Mycobacteria in 3 cultures | Mycobacteria |
CoNS = coagulase-negative staphylococci.
Treatment of Pseudocyst
The most common initial surgical treatments of pseudocyst were shunt externalization (90/141, 63.8%) or complete removal and EVD placement (42/141, 29.8%) (Table 1). Externalization failed in 9/90 (10%) children with persistently positive cultures; their shunts were removed and EVDs were placed. The median duration of externalization or EVD was 10 days (IQR 7, 14 days), and all patients had negative CSF cultures documented before shunt reimplantation.
As previously mentioned, 6 children had a shunt revision only without removal or externalization on initial diagnosis, and all experienced reoperation for shunt failure within 30 days. Among 3 children who had the shunt immediately placed into the peritoneal cavity, 2 shunts failed because of infection and 1 required revision. The other 3 children had the shunt moved to a nonperitoneal site; shunts failed in 2, requiring revision, and 1 child experienced infection. Two of the 6 patients—1 with positive CSF culture (Streptococcus viridans) and 1 with a positive APC culture (acid-fast bacilli)—were treated with antibiotics. Both shunts were revised to a nonperitoneal location. The patient with a positive APC culture had a subsequent infection. The child with a positive CSF culture required future shunt revision (1 culture positive, following day negative).
Shunt Reimplantation Site
The reimplantation/implantation site at the end of APC treatment was equally distributed between the peritoneum (70/141) and a nonperitoneal site (71/141). To understand the surgeons’ rationale for this decision, we compared patient and treatment factors between the peritoneal and nonperitoneal groups (Table 1). There were no significant differences in most patient and treatment factors. The children who had their shunt reimplanted in the abdomen were slightly younger (8.4 vs 10.6 years, p = 0.018). No association was found between HCRN site and shunt implantation location after APC treatment (p = 0.218).
When the last procedure before APC diagnosis was a distal shunt revision, shunts were more commonly implanted in a nonperitoneal location at the end of APC treatment (45.8% vs 10.8%, p = 0.002). Conversely, when the APC was treated with total removal and EVD placement, more shunts were reimplanted in the abdomen (41.4% vs 18.3%, p = 0.006). For children whose shunts were reimplanted peritoneally, the most common method used was minilaparotomy (52.9%), followed by laparoscope-assisted (28.6%) or trocar-assisted (15.7%) laparotomy and use of a previous tract (2.9%).
Complications occurred in 5 children prior to discharge, including positive CSF culture, deep vein thrombosis, visual disturbance, pneumothorax, and seizure (1 each).
Shunt Failure After Reimplantation
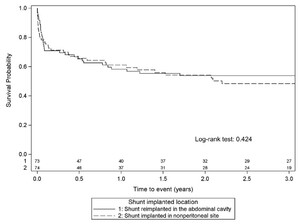
The overall shunt failure rate after APC treatment was 49%. The median and absolute number of revisions did not differ between cohorts at 6 (n = 138, p = 0.711), 12 (n = 135, p > 0.99), and 24 (n = 131, p = 0.860) months after reimplantation (Table 3). Ten children whose shunt was reimplanted into the abdominal cavity experienced a recurrent APC (14.3%). More children whose shunt was reimplanted in the abdominal cavity had a subsequent shunt infection (25.7% vs 7.0%, p = 0.003), whereas more children whose shunt was implanted at a nonperitoneal site had a subsequent noninfectious shunt revision (42.3% vs 22.9%, p = 0.019). Of the 70 patients who had the shunt reimplanted in the abdominal cavity, 18 had subsequent infections: 9 (50%) were APC and the rest were not. Shunt survival after APC treatment did not appear to be associated with initial surgical treatment (Fig. 2) or reimplantation site (Fig. 3).
Outcomes after shunt reimplantation
| Variable | Shunt Reimplantation Site | p Value | |
|---|---|---|---|
| Abdominal Cavity, n = 70 | Nonperitoneal Site, n = 71 | ||
| Revisions after reimplantation | |||
| 在6 w /金属氧化物半导体 | 19/68 (27.9%); 0 [0, 1] | 22/70 (31.4%); 0 [0, 1] | 0.711 |
| w/in 12 mos | 24/67 (35.8%); 0 [0, 1] | 25/68 (36.8%); 0 [0, 1] | >0.99 |
| w/in 24 mos | 27/67 (40.3%); 0 [0, 1] | 27/64 (42.2%); 0 [0, 1] | 0.860 |
| Recurrent APC | 10/70 (14.3%) | NA | NA |
| Shunt failure after APC | <0.001 | ||
| Revision | 16/70 (22.9%) | 30/71 (42.3%) | 0.019 |
| Infection | 18/70 (25.7%) | 5/71 (7.0%) | 0.003 |
| No failure | 36/70 (51.4%) | 36/71 (50.7%) | |
NA = not applicable.
Values are expressed as number (%) or median [Q1, Q3]. The p values were calculated using Fisher’s exact test. Boldface type indicates statistical significance.

Kaplan-Meier survival curve based on initial APC surgical management technique.

Kaplan-Meier survival curve for shunt implantation based on site (peritoneal vs extraperitoneal site) after APC diagnosis and management.
On univariable analysis, younger age at APC diagnosis (8.3 vs 12.2 years, p = 0.006) and shunt surgery within 12 weeks before APC diagnosis (59.5% vs 40.5%, p = 0.012) were associated with overall shunt failure after APC treatment (Table 4). In addition, a longer duration of antibiotic treatment appeared to be associated with a higher risk of shunt failure, but the HR of 1.01 suggests that this is not clinically impactful. An etiology of myelomeningocele compared with other etiologies had insignificantly lower rates of subsequent failure after APC treatment (p = 0.089) (Table 4). For those patients whose shunt was reimplanted into the abdomen, the method of reimplantation influenced the subsequent failure rate after APC treatment. Children who underwent trocar placement were more likely to experience failure (HR 5.44 [95% CI 1.80–16.41]) than those who underwent laparoscopic placement (25% vs 75%, p = 0.001); however, the number of children for whom a trocar entry was used was small (n = 11). Slightly more children who underwent a minilaparotomy went on to require revision (54.1% vs 45.9%).
Univariable Cox proportional hazards model for shunt failure (revision/infection)
| Variable | No Shunt Failure | Shunt Failure | HR (95% CI) | p Value |
|---|---|---|---|---|
| Age at APC Dx, yrs | 12.2 [6.2, 15.7] | 8.3 [2.8, 13.0] | 0.94 (0.90–0.98) | 0.006 |
| Etiology of hydrocephalus | 0.089 | |||
| Post-pIVH | 27 (41.5%) | 38 (58.5%) | Reference | |
| Myelomeningocele | 19 (63.3%) | 11 (36.7%) | 0.49 (0.25–0.97) | |
| Other etiology | 26 (56.5%) | 20 (43.5%) | 0.69 (0.40–1.20) | |
| Gastrostomy | 12 (52.2%) | 11 (47.8%) | 0.98 (0.52–1.88) | 0.963 |
| Complex chronic conditions | 45 (51.7%) | 42 (48.3%) | 0.91 (0.56–1.48) | 0.701 |
| Shunt implant location | 0.425 | |||
| Abdominal cavity | 36 (51.4%) | 34 (48.6%) | 0.82 (0.51–1.33) | |
| Extraperitoneal site | 36 (50.7%) | 35 (49.3%) | Reference | |
| Mos from last shunt op to APC formation | 4.4 [1.5, 29.8] | 3.3 [1.5, 15.5] | 0.99 (0.97–1.00) | 0.162 |
| Previous ETV/ETV-CPC | 6 (54.5%) | 5 (45.5%) | 0.86 (0.34–2.13) | 0.736 |
| Previous subgaleal shunt | 2 (28.6%) | 5 (71.4%) | 2.33 (0.94–5.82) | 0.069 |
| Previous shunt surgery | 0.344 | |||
| Revision | 31 (50.8%) | 30 (49.2%) | Reference | |
| Infection | 3 (33.3%) | 6 (66.7%) | 1.89 (0.79–4.57) | |
| New shunt placement | 7 (43.8%) | 9 (56.3%) | 1.26 (0.60–2.65) | |
| Previous revision type | 0.364 | |||
| Not revised | 33 (55.9%) | 26 (44.1%) | 0.97 (0.49–1.89) | |
| Proximal | 16 (55.2%) | 13 (44.8%) | Reference | |
| Distal | 8 (44.4%) | 10 (55.6%) | 1.51 (0.66–3.48) | |
| Both | 15 (42.9%) | 20 (57.1%) | 1.51 (0.75–3.06) | |
| Previous peritoneal entry method | 0.628 | |||
| Minilaparotomy | 14 (45.2%) | 17 (54.8%) | Reference | |
| Laparoscopic | 3 (33.3%) | 6 (66.7%) | 1.85 (0.72–4.78) | |
| Trocar | 2 (40.0%) | 3 (60.0%) | 1.53 (0.44–5.30) | |
| Previous tract used w/o other methods | 3 (100.0%) | 0 (0.0%) | 0.00 | |
| Unknown/not revised | 13 (56.5%) | 10 (43.5%) | 0.87 (0.40–1.90) | |
| Shunt surgery w/in previous 12 wks | 15 (40.5%) | 22 (59.5%) | 1.92 (1.15–3.20) | 0.012 |
| Tx of infection | 0.845 | |||
| Externalization | 44 (48.9%) | 46 (51.1%) | Reference | |
| Externalization & subsequent removal | 6 (66.7%) | 3 (33.3%) | 0.71 (0.22–2.28) | |
| Surgical removal | 22 (52.4%) | 20 (47.6%) | 1.00 (0.59–1.69) | |
| Pseudocyst culture positive from initial APC Tx | 10 (50.0%) | 10 (50.0%) | 1.43 (0.73–2.80) | 0.300 |
| CSF culture positive from initial APC Tx | 12 (54.5%) | 10 (45.5%) | 1.16 (0.59–2.27) | 0.665 |
| Pseudocyst or CSF culture positive from initial APC Tx | 14 (56.0%) | 11 (44.0%) | 1.11 (0.58–2.13) | 0.742 |
| Duration of EVD/externalization prior to reimplantation, days | 9.5 [7.0, 14.0] | 11.0 [7.0, 14.0] | 1.01 (1.00–1.02) | 0.019 |
| Complex procedure | 5 (55.6%) | 4 (44.4%) | 1.12 (0.39–3.22) | 0.832 |
| Programmable valve | 18 (48.6%) | 19 (51.4%) | 1.06 (0.61–1.84) | 0.841 |
| No. of previous shunt surgeries* | 2.0 [1.0, 3.0] | 2.0 [1.0, 5.0] | 1.05 (0.95–1.17) | 0.317 |
CPC = choroid plexus cauterization.
Unless otherwise indicated, values are expressed as number (%) or median [Q1, Q3]. Boldface type indicates statistical significance.
Only included children who had a primary procedure in the registry.
Multivariable modeling demonstrated that undergoing a shunt procedure within 12 weeks before APC diagnosis (HR 1.79 [95% CI 1.04–3.07], p = 0.035) was the only factor independently associated with subsequent failure after APC treatment. Younger age at APC diagnosis (HR 0.96 [95% CI 0.92–1.00], p = 0.076) was suggestive of subsequent failure but the difference was not statistically significant.
Discussion
In this multicenter cohort study, we compared treatment methods and outcomes for shunt reimplantation in children who were diagnosed with an APC. This is the largest study of APCs in children and the only study thus far comparing treatment strategies. We found that the CSF Gram stain was positive in 10.3% of children, 14.2% of APC cultures were positive, and 15.6% of CSF cultures were positive. No differences were found in overall shunt failure rate either between initial management strategies of shunt removal/EVD placement versus shunt externalization or between reimplantation in the abdominal cavity and implantation in nonperitoneal sites. However, implantation of the distal catheter at a nonperitoneal site was associated with a higher risk of noninfectious shunt revision, which needs to be balanced against a higher risk of shunt infection found in peritoneal reimplantation. We also found that children who had shunt surgery within 12 weeks of APC diagnosis were 1.79 times more likely to have shunt failure after APC treatment.
Previous histopathological studies have shown that pseudocyst walls consist of a peritoneal serous membrane with chronic inflammatory features. Although the cause of APC is unclear, a sterile or infectious inflammatory process has been posited,2,8,9which ultimately leads to a surface that does not allow for CSF absorption.4Most cultures in this series were negative, which suggests an inflammatory component rather than an infectious one.
The presence of an APC is indicative of infection by the HCRN definition, an assertion supported by many previous studies.2,10,11In this study, however, only 18% of patients had positive APC or CSF cultures. Dabdoub et al.2found that 33% of patients had a positive CSF culture on presentation, and culture positivity was more common among younger children; their study did not comment on APC fluid culture results. Similar to Gmeiner et al.,3we found that the infection rate of APC fluid was 14%. We found infection as the cause of the next failure after APC treatment in 16% of children overall. Moreover, in the cohort of children whose shunts were reimplanted in the abdominal cavity, the next shunt failure or intervention was more likely to be secondary to infection. In any study of APC, including this one, the infection rate may be artificially lower than expected because of the presence of slower-growing bacteria, the importance of which has previously been described.2,8,10,11It has also been suggested that sending the tip of the peritoneal catheter for culture may increase the rates of infection detection,12but this is not commonplace and not standardized among HCRN centers. We found that gram-positive organisms were most common on the cultures examined (Table 2). Historically, CSF culture results in the setting of APC wereStaphylococcus epidermidisandS. aureus.2In this study 15/22 cultures containedStaphylococcusspecies, 4 of which wereS. aureus.
Among 14 patients, both adults and children, Gmeiner et al.3observed a 50% shunt infection rate, with APC fluid returning positive in 3 patients; no association was found between time to APC and first surgical procedure or first peritoneal catheter, type of first surgical procedure, or cause of hydrocephalus.
The number of shunt revisions is thought to be a risk factor for APC formation.2,5Hahn et al.13reported a mean of 11.2 shunt operations in patients with APC. Similarly, Rainov et al.5found that 50% of patients with APC had between 5 and 10 shunt revisions. Our cohort of young children demonstrated a much lower median number—1 previous shunt revision before APC diagnosis—suggesting an index of suspicion should remain in all children. Multivariable modeling demonstrated that children who underwent shunt surgery within 12 weeks of APC diagnosis were 1.79 times more likely to experience shunt failure after APC treatment, which should prompt closer follow-up in this group. On the basis of our data, we advocate that children of younger age should be monitored carefully for shunt failure after APC treatment as well, even though this factor was not confirmed to be an independent predictor.
Previous shunt infection has also been considered a risk factor for APC development.6在我们的群体中,只有11%的病人接受了回避t reimplantation for infection before APC diagnosis, and multivariate analysis did not identify previous shunt infection as an independent predictor for failure after APC management. The peritoneal entry method for the shunt operation immediately preceding APC diagnosis was more commonly a minilaparotomy in the group that ultimately underwent reimplantation in the abdominal cavity, with only 4.9% having undergone laparoscopic assistance (vs 23% in the extraperitoneal site group). This may indicate more use of laparoscopy in the complex or hostile abdomen cohort, which contributed to abandonment of the peritoneal cavity. Interestingly, the method of shunt replacement into the abdomen did influence the subsequent failure rate. Specifically, shunts reimplanted in the abdomen using trocar guidance were 5.44 times more likely to fail than those placed laparoscopically. However, this finding should be interpreted carefully, because the number of children who underwent trocar entry was small (n = 11).
There did not seem to be a particular variable that led to the decision to replace the shunt in one location vs another, other than age. The patients whose shunts were reimplanted in the abdomen were younger, which reflects the reluctance to use nonperitoneal sites in young children. One might expect that comorbidities, gastrostomy tube presence, or positive APC cultures might impact the decision, and yet none of these were different (Table 1). No difference was found in overall shunt failure rates between reimplantation in the abdomen and implantation in nonperitoneal sites, although noninfectious revisions were more likely with nonperitoneal placement and infection was more likely to occur in those replaced into the abdomen after APC treatment. As previously described, CSF shunt infections are a substantial issue for children experiencing hydrocephalus. Shunt infection is responsible for more than 2400 admissions and 59,000 hospital days annually in the US alone.14Its treatment involves at least 2 surgical procedures and intravenous antibiotics,15and reinfection rates are as high as 25%.16,17Shunt infections account for more cost, hospital days, and charges than shunt revisions.14Given the juxtaposition of higher rate of revision at nonperitoneal sites and higher infection rate at the peritoneal site, perhaps a formal cost-effectiveness study is appropriate to determine the best policy for selecting a reimplantation site.
Although the use of antibiotic-impregnated catheters has been proven to be efficacious in infection prevention or reduction in pediatric hydrocephalus,18以前的在这个研究th分流手术e use of antibiotic-impregnated catheters in only 17 cases (data on antibiotic-impregnated catheter use were not collected until November 2016). Thus, no definitive recommendations can be made regarding the use of these catheters in terms of APC formation or prevention.
Despite the large size of this cohort study, the cause of APC remains unknown, although it is posited to be related to infection, hypersensitivity, or a possible sterile inflammatory hyperstimulus causing degradation of shunt material and formation of the pseudocyst. This study did not investigate risk factors for APC development. The prevalence of gastrostomy tube was higher than in previous HCRN investigations for first CSF shunt infection6and shunt malfunction,19but our analysis did not identify an association between having a gastrostomy tube and shunt failure after APC treatment, which suggests that future investigation of risk factors for APC development itself is necessary. In the HCRN, the presence of an APC is considered to be a manifestation of shunt infection, and yet only 14% of APC and 15.6% of CSF culture results in this study were positive. This low rate of positive cultures may have occurred because 1) there was no minimum required duration of cultures to grow, so slow-growing organisms may have been missed; 2) the HCRN definition of APC may capture noninfected loculated fluid collections; and/or 3) a shunt tap when infection is suspected may identify organisms in the CSF for which the child is treated without undergoing abdominal imaging, so that an associated APC might be missed. Nevertheless, the high rate of recurrent infection and shunt failure with revision alone suggests that treatment with antibiotics plus shunt externalization or removal and EVD is reasonable, an approach that is also supported in the literature.19For initial APC management, externalization only failed in 10% of children, and thus seems reasonable as an initial approach.
Limitations
The study is derived from a registry of patients treated surgically for hydrocephalus. The current cohort includes those entered in the registry prospectively, but some children are missing data from the previous shunt surgery. With only 25 subjects having positive cultures and information on previous shunt surgery available in only 86 children, we could not analyze risk factors for culture positivity from this data set because any results would be lacking statistical power. The data were collected with protocols for fidelity, validation, and quality control. The HCRN includes centers in North America only, so the findings, specifically the microbiology results, should be interpreted carefully on a global level. As with any surgical study, the treatment of APC was subject to the bias of the treating surgeon; there is currently no protocol in place in the HCRN to manage APC, which was the impetus for this investigation. The definition of APC through the HCRN is a limitation as well because it is not inclusive or exclusive of infection but is automatically treated as such regardless of culture positivity.
There is no set length of time that each institution allows for growth before disposing of the cultured material. The HCRN data collection requires that cultures marked negative must be final in the electronic medical record, but this does not dictate how long the sites themselves hold on to the culture. The median length of time to reporting of positive cultures was 4 days, but the date/duration of negative cultures was not recorded. Indolent organisms may take up to 14 days to grow and therefore may be underestimated in our data. The use of 16S ribosomal RNA to identify the microbiota in CSF shunt infection has been described but is not commonplace or standard practice among HCRN centers. Given the low positivity of traditional cultures in this report, the adoption of this technique in cases of APC with/without high clinical suspicion could be considered.20,21Additionally, the use of antibiotic-coated EVDs is not standard among centers, which may also influence the findings of culture positivity. Duration of antibiotic treatment, method of treatment, adherence to Infectious Diseases Society of America recommendations, and prolonged postsurgical antibiotics were not captured in this study and may play a role, although all patients had documented negative cultures before shunt reimplantation.
Conclusions
The management of APC in children with shunted hydrocephalus is variable. APC is usually managed with externalization or shunt removal with EVD placement; shunt revision without removal led to failure within 30 days. APC cultures were positive in 14.2% of children, CSF cultures were positive in 15.6% of patients, and 17.7% of either APC or CSF cultures were positive overall. Shunt surgery in the 12 weeks preceding APC diagnosis was associated with failure after APC treatment; younger age at APC diagnosis was also associated with subsequent failure. Although no differences were found in the overall shunt failure rate between reimplantation in the abdominal cavity and implantation in nonperitoneal sites, infection was a much more common reason for failure after reimplantation in the abdomen, and noninfectious shunt revisions were more common in the nonperitoneal distal catheter sites.
Acknowledgments
HCRN感谢以下的来源funding: NINDS (grant nos. 1RC1NS068943-01 Challenge, and 1U01NS107486-01A1 ESTHI); private philanthropy; and the Hydrocephalus Association. None of the sponsors participated in design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of this paper. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the sponsors.
We thank our colleagues for their past and ongoing support of HCRN: D. Brockmeyer, M. Walker, R. Bollo, S. Cheshier, R. Iyer, J. Blount, J. Johnston, B. Rocque, L. Acakpo-Satchivi, W.J. Oakes, P. Dirks, G. Ibrahim, J. Rutka, M. Taylor, D. Curry, G. Aldave, R. Dauser, A. Jea, S. Lam, H. Weiner, T. Luerssen, R. Ellenbogen, J. Ojemann, A. Lee, A. Avellino, S. Greene, M. McDowell, E. Tyler-Kabara, R. Kellogg, T. Abel, T.S. Park, J. Strahle, J. Roland, S. McEvoy, M. Smyth, N. Tulipan, F. Haji, A. Singhal, P. Steinbok, D. Cochrane, W. Hader, C. Gallagher, M. Benour, P. Chiarelli, S. Durham, E. Kiehna, J.G. McComb, A. Robison, A. Alexander, M. Handler, B. O’Neill, C. Wilkinson, L. Governale, A. Drapeau, J. Leonard, E. Sribnick, A. Shaikhouni, E. Ahn, A. Cohen, M. Groves, S. Robinson, C.M. Bonfield, and C. Shannon. In addition, our work would not be possible without the outstanding support of the dedicated personnel at each clinical site and the data coordinating center. Special thanks go to: A. Ludwick, L. Holman, J. Clawson, P. Martello, N. Tattersall, T. Bach (Salt Lake City); T. Caudill, P. Komarova, A. Arynchyna, A. Bey (Birmingham); N. Emami, H. Ashrafpour, M. Lamberti-Pasculli, L. O’Connor (Toronto); E. Santisbon, E. Sanchez, S. Martinez, S. Ryan (Houston); H. Willis, K. Hall, C. Gangan, J. Klein, A. Anderson, G. Bowen (Seattle); S. Thambireddy, K. Diamond, A. Luther (Pittsburgh); A. Morgan, H. Botteron, D. Morales, M. Gabir, D. Berger, D. Mercer (St. Louis); M. Stone, A. Wiseman, J. Stoll, D. Dawson, S. Gannon (Nashville); I. Watson, A. Cheong, R. Hengel (British Columbia); R. Rashid, S. Ahmed (Calgary); J. Yea, A. Loudermilk (Baltimore); H. Berroya, N. Chapman, N. Rea, C. Cook (Los Angeles); S. Staulcup (Colorado); J. Haught, H. Lehmann, S. Saraswat, A. Sheline (Columbus); and N. Nunn, M. Langley, V. Wall, D. Austin, B. Conley, V. Freimann, L. Herrera, B. Miller (Utah Data Coordinating Center).
We thank Kristin Kraus for editorial assistance.
Appendix
HCRN Members
The HCRN currently consists of the following sites and investigators: Primary Children’s Hospital, University of Utah (J. Kestle); Children’s of Alabama, University of Alabama at Birmingham (C. Rozzelle, B. Rocque); Hospital for Sick Children, University of Toronto (J. Drake, A. Kulkarni); Texas Children’s Hospital, Baylor College of Medicine (W. Whitehead); Seattle Children’s Hospital, University of Washington (S. Browd, J. Hauptman); Children’s Hospital of Pittsburgh, University of Pittsburgh (I. Pollack); St. Louis Children’s Hospital, Washington University in St. Louis (D. Limbrick); Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt University Medical Center (J. Wellons, R. Naftel); British Columbia Children’s Hospital, University of British Columbia (M. Tamber); Alberta Children’s Hospital, University of Calgary (J. Riva-Cambrin); The Johns Hopkins Hospital (E. Jackson); Children’s Hospital of Los Angeles (M. Krieger, J. Chu, T. Simon); Children’s Hospital Colorado (T. Hankinson); Nationwide Children’s Hospital (J. Pindrik); University of Manitoba (P. McDonald); HCRN Data Coordinating Center, Department of Pediatrics, University of Utah (R. Holubkov).
Disclaimer
The views expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the US Government.
Disclosures
Dr. Hankinson reported a consultant fee for an educational course from Monteris outside the submitted work. Dr. Hauptman reported personal fees from Medtronic and BK Medical outside the submitted work.
Author Contributions
Conception and design: Kestle, Ravindra, Wellons, Hankinson, Kulkarni, Rozzelle, Krieger, Nunn. Acquisition of data: Kestle, Jensen, Riva-Cambrin, Limbrick, Pindrik, Jackson, Hauptman, Tamber, Kulkarni, Rocque, Whitehead, Simon, McDonald, Nunn. Analysis and interpretation of data: Kestle, Ravindra, Jensen, Riva-Cambrin, Wellons, Jackson, Kulkarni, Rocque, Rozzelle, Krieger, Reeder. Drafting the article: Kestle, Ravindra, Jensen, Riva-Cambrin, Hankinson. Critically revising the article: Kestle, Ravindra, Riva-Cambrin, Wellons, Limbrick, Pindrik, Jackson, Pollack, Hankinson, Hauptman, Tamber, Kulkarni, Rocque, Whitehead, Chu, Krieger, Simon, McDonald, Nunn. Reviewed submitted version of manuscript: Kestle, Ravindra, Jensen, Riva-Cambrin, Wellons, Limbrick, Pindrik, Jackson, Pollack, Hankinson, Hauptman, Tamber, Kulkarni, Rocque, Rozzelle, Whitehead, Krieger, Simon, McDonald, Nunn. Approved the final version of the manuscript on behalf of all authors: Kestle. Statistical analysis: Jensen, Reeder. Administrative/technical/material support: Riva-Cambrin, Pollack, Krieger, Nunn. Study supervision: Kestle, Krieger, Nunn.
补充信息
Previous Presentations
This work was presented at the AANS/CNS Section on Pediatric Neurological Surgery in Washington, D.C., December 1–4, 2022.
References
-
1 ↑
ReddyGK,BollamP,CalditoG.Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus.World Neurosurg.2014;81(2):404–410.
-
2 ↑
DabdoubCB,DabdoubCF,ChavezM,et al.Abdominal cerebrospinal fluid pseudocyst: a comparative analysis between children and adults.Childs Nerv Syst.2014;30(4):579–589.
-
3 ↑
GmeinerM,WagnerH,van OuwerkerkWJR,SenkerW,HollK,GruberA.Abdominal pseudocysts and peritoneal catheter revisions: surgical long-term results in pediatric hydrocephalus.World Neurosurg.2018;111:e912–e920.
-
4 ↑
de OliveiraRS,BarbosaA,VicenteYA,MachadoHR.An alternative approach for management of abdominal cerebrospinal fluid pseudocysts in children.Childs Nerv Syst.2007;23(1):85–90.
-
5 ↑
RainovN,SchobessA,HeideckeV,BurkertW.Abdominal CSF pseudocysts in patients with ventriculo-peritoneal shunts. Report of fourteen cases and review of the literature.Acta Neurochir (Wien).1994;127(1-2):73–78.
-
6 ↑
SimonTD,ButlerJ,WhitlockKB,et al.Risk factors for first cerebrospinal fluid shunt infection: findings from a multi-center prospective cohort study.J Pediatr.2014;164(6):1462–8.e2.
-
7 ↑
TamberMS,KestleJRW,ReederRW,et al.Temporal trends in surgical procedures for pediatric hydrocephalus: an analysis of the Hydrocephalus Clinical Research Network Core Data Project.J Neurosurg Pediatr.2020;27(3):269–276.
-
8 ↑
MobleyLWIII,DoranSE,HellbuschLC.Abdominal pseudocyst: predisposing factors and treatment algorithm.Pediatr Neurosurg.2005;41(2):77–83.
-
9 ↑
KariyattilR,SteinbokP,SinghalA,CochraneDD.Ascites and abdominal pseudocysts following ventriculoperitoneal shunt surgery: variations of the same theme.J Neurosurg.2007;106(5)(suppl):350–353.
-
10 ↑
HanakBW,BonowRH,HarrisCA,BrowdSR.Cerebrospinal fluid shunting complications in children.Pediatr Neurosurg.2017;52(6):381–400.
-
11 ↑
RoitbergBZ,TomitaT,McLoneDG.Abdominal cerebrospinal fluid pseudocyst: a complication of ventriculoperitoneal shunt in children.Pediatr Neurosurg.1998;29(5):267–273.
-
12 ↑
SalomãoJF,LeibingerRD.Abdominal pseudocysts complicating CSF shunting in infants and children. Report of 18 cases.Pediatr Neurosurg.1999;31(5):274–278.
-
13 ↑
Hahny,恩格尔哈德H,McLoneDG.Abdominal CSF pseudocyst Clinical features and surgical management.Pediatr Neurosci.1985-1986;12(2):75-79.
-
14 ↑
SimonTD,Riva-CambrinJ,SrivastavaR,BrattonSL,DeanJM,KestleJR.Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths.J Neurosurg Pediatr.2008;1(2):131–137.
-
15 ↑
WhiteheadWE,KestleJR.The treatment of cerebrospinal fluid shunt infections. Results from a practice survey of the American Society of Pediatric Neurosurgeons.Pediatr Neurosurg.2001;35(4):205–210.
-
16 ↑
KulkarniAV,RabinD,Lamberti-PasculliM,DrakeJM.Repeat cerebrospinal fluid shunt infection in children.Pediatr Neurosurg.2001;35(2):66–71.
-
17 ↑
KestleJR,GartonHJ,WhiteheadWE,et al.Management of shunt infections: a multicenter pilot study.J Neurosurg.2006;105(3)(suppl):177–181.
-
18 ↑
ChuJ,JensenH,HolubkovR,et al.The Hydrocephalus Clinical Research Network quality improvement initiative: the role of antibiotic-impregnated catheters and vancomycin wound irrigation.J Neurosurg Pediatr.2022;29(6):711–718.
-
19 ↑
Riva-CambrinJ,KestleJR,HolubkovR,et al.Risk factors for shunt malfunction in pediatric hydrocephalus: a multicenter prospective cohort study.J Neurosurg Pediatr.2016;17(4):382–390.
-
20 ↑
SimonTD,PopeCE,BrowdSR,et al.Evaluation of microbial bacterial and fungal diversity in cerebrospinal fluid shunt infection.PLoS One.2014;9(1):e83229.
-
21 ↑
WhitlockKB,PopeCE,HodorP,et al.Characterization of cerebrospinal fluid (CSF) microbiota from patients with CSF shunt infection and reinfection using high throughput sequencing of 16S ribosomal RNAgenes.PLoS One.2021;16(1):e0244643.